Copper-dependent halogenase catalyses unactivated C−H bond functionalization
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
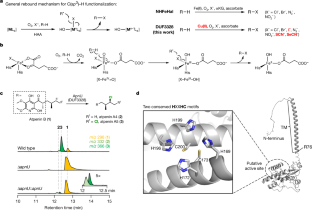
Carbon–hydrogen (C–H) bonds are the foundation of essentially every organic molecule, making them an ideal place to do chemical synthesis. The key challenge is achieving selectivity for one particular C(sp3)−H bond1–3. In recent years, metalloenzymes have been found to perform C(sp3)−H bond functionalization4,5. Despite substantial progresses in the past two decades6,7, enzymatic halogenation and pseudohalogenation of unactivated C(sp3)−H—providing a functional handle for further modification—have been achieved with only non-haem iron/α-ketoglutarate-dependent halogenases, and are therefore limited by the chemistry possible with these enzymes8. Here we report the discovery and characterization of a previously unknown halogenase ApnU, part of a protein family containing domain of unknown function 3328 (DUF3328). ApnU uses copper in its active site to catalyse iterative chlorinations on multiple unactivated C(sp3)−H bonds. By taking advantage of the softer copper centre, we demonstrate that ApnU can catalyse unprecedented enzymatic C(sp3)−H bond functionalization such as iodination and thiocyanation. Using biochemical characterization and proteomics analysis, we identified the functional oligomeric state of ApnU as a covalently linked homodimer, which contains three essential pairs—one interchain and two intrachain—of disulfide bonds. The metal-coordination active site in ApnU consists of binuclear type II copper centres, as revealed by electron paramagnetic resonance spectroscopy. This discovery expands the enzymatic capability of C(sp3)−H halogenases and provides a foundational understanding of this family of binuclear copper-dependent oxidative enzymes. A halogenase enzyme uses the copper in its active site to catalyse iterative chlorinations on multiple unactivated carbon−hydrogen bonds, enabling carbon−hydrogen functionalization that is not achievable with an iron-based halogenase.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


