Methanol transfer supports metabolic syntrophy between bacteria and archaea
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
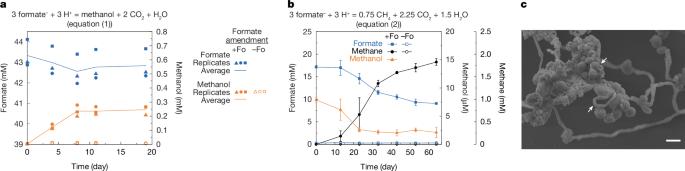
In subsurface methanogenic ecosystems, the ubiquity of methylated-compound-using archaea—methylotrophic methanogens1–4—implies that methylated compounds have an important role in the ecology and carbon cycling of such habitats. However, the origin of these chemicals remains unclear5,6 as there are no known energy metabolisms that generate methylated compounds de novo as a major product. Here we identified an energy metabolism in the subsurface-derived thermophilic anaerobe Zhaonella formicivorans7 that catalyses the conversion of formate to methanol, thereby producing methanol without requiring methylated compounds as an input. Cultivation experiments showed that formate-driven methanologenesis is inhibited by the accumulation of methanol. However, this limitation can be overcome through methanol consumption by a methylotrophic partner methanogen, Methermicoccus shengliensis. This symbiosis represents a fourth mode of mutualistic cross-feeding driven by thermodynamic necessity (syntrophy), previously thought to rely on transfer of hydrogen, formate or electrons8–10. The unusual metabolism and syntrophy provide insights into the enigmatic presence of methylated compounds in subsurface methanogenic ecosystems and demonstrate how organisms survive at the thermodynamic limit through metabolic symbiosis. Zhaonella formicivorans produces methanol, for a novel syntrophic interaction, without requiring methylated compounds as an input.
甲醇转移支持细菌和古生菌之间的代谢共生
在地下产甲烷生态系统中,利用甲基化化合物的古生甲烷菌1,2,3,4的普遍存在表明甲基化化合物在这些栖息地的生态和碳循环中具有重要作用。然而,这些化学物质的来源仍然不清楚,因为没有已知的能量代谢产生甲基化化合物作为主要产物。在这里,我们发现了地下来源的嗜热厌氧菌甲酸兆霉7的能量代谢,它催化甲酸转化为甲醇,从而产生甲醇,而不需要甲基化化合物作为输入。培养实验表明,甲酸驱动的甲醇生成受到甲醇积累的抑制。然而,这一限制可以通过甲基营养伙伴产甲烷菌——生利热微球菌的甲醇消耗来克服。这种共生关系代表了由热力学必要性驱动的第四种互食模式(同质),以前认为它依赖于氢、甲酸或电子的转移8,9,10。这种不寻常的代谢和共生关系为地下产甲烷生态系统中甲基化化合物的神秘存在提供了见解,并展示了生物体如何通过代谢共生在热力学极限下生存。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


