Optimization of pyrazole/1,2,4-triazole as dual EGFR/COX-2 inhibitors: Design, synthesis, anticancer potential, apoptosis induction and cell cycle analysis
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
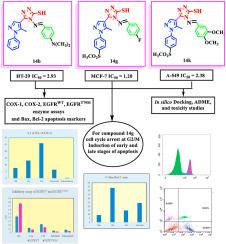
A novel series of pyrazol-4-yl-1,2,4-triazole-3-thiol derivatives 14a-l was designed, prepared and characterized by many spectroscopic techniques. All the novel compounds were screened for their anti-proliferative activity towards breast cancer cell line (MCF-7), colon cancer cell line (HT-29) and lung cancer cell line (A-549) utilizing celecoxib, erlotinib and osimertinib as standards. Compounds 14b, 14g and 14k were the most active towards HT-29, MCF-7 and A-549 cell lines, sequentially with IC50 = 1.20-2.93 μM compared with celecoxib (IC50 = 16.47-41.45 μM), erlotinib (IC50 = 1.95-33.57 μM) and osimertinib (IC50 = 0.75-3.45 μM). These most active derivatives 14b, 14g and 14k were further investigated for their inhibitory potential against COX and EGFR enzymes. These compounds 14b, 14g and 14k suppressed COX-2 (IC50 = 0.560-4.692 μM), EGFRWT (IC50 = 0.121-0.423 μM) and EGFRT790M (IC50 = 0.076-0.764 μM) enzymes. Compounds 14b, 14g and 14k displayed apoptosis induction by up-regulating Bax and down-regulating Bcl-2 protein levels. Cell cycle analysis recorded that exposure of MFC-7 cells to compound 14g resulted in a significant increase in the percentage of cells at the G2/M to 39.15% compared to the standard erlotinib (9.87%). Docking study of the most potent candidates 14b, 14g and 14k within COX-2, EGFRWT and EGFRT790M active regions was conducted to suggest the binding mode of these compounds inside these target enzymes.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


