Cytotoxic pyrrole-based gold(III) chelates target human topoisomerase II as dual-mode inhibitors and interact with human serum albumin
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
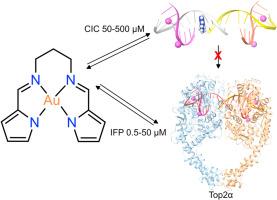
Topoisomerase IIα (Top II) is a critical enzyme that resolves DNA topology during transcription and replication. Inhibitors of Top II are used as anticancer agents and are classified as interfacial poisons (IFPs) or catalytic inhibitors (CICs). Here, we report a novel class of cytotoxic, stable cationic gold(III) Schiff base chelates (AuL1, AuL2, and AuL3) with DNA-intercalating properties. In the NCI-60 screen, AuL1 and AuL3 exhibited potent cytotoxicity (mean GI50 values of 11 (7) μM and 14 (9) μM, respectively), whereas AuL2 showed minimal cytotoxicity. Cluster analysis aligned AuL1 and AuL3 with the Top II poison etoposide. Mechanistic studies revealed that AuL1 acts as an IFP at concentrations between 0.5–50 μM and as a CIC at concentrations between 50–500 μM. Further investigations demonstrated that all three gold(III) chelates bind to and intercalate DNA, the main substrate for Top II. Finally, binding studies with human serum albumin (HSA) indicated that the chelates have moderate affinity for the protein. Thermodynamic analysis indicates entropically driven binding, with minimal structural disruption observed via UV-CD spectroscopy. These findings highlight the dual mode Top II inhibition mechanism delineated for the gold(III) chelates and their favourable pharmacodynamic interactions with HSA, underscoring their potential as promising anticancer agents.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


