Resolving the three-dimensional interactome of human accelerated regions during human and chimpanzee neurodevelopment
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Human accelerated regions (HARs) have been implicated in human brain evolution. However, insight into the genes and pathways they control is lacking, hindering the understanding of their function. Here, we identify 2,963 conserved gene targets for 1,590 HARs and their orthologs in human and chimpanzee neural stem cells (NSCs). Conserved gene targets are enriched for neurodevelopmental functions and are overrepresented among differentially expressed genes (DEGs) identified in human NSCs (hNSCs) and chimpanzee NSCs (cNSCs) as well as in human versus non-human primate brains. Species-specific gene targets do not converge on any function and are not enriched among DEGs. HAR targets also show cell-type-specific expression in the human fetal brain, including in outer radial glia, which are linked to cortical expansion. Our findings support that HARs influence brain evolution by altering the expression of ancestral gene targets shared between human and chimpanzee rather than by gaining new targets in human and facilitate hypothesis-directed studies of HAR biology.
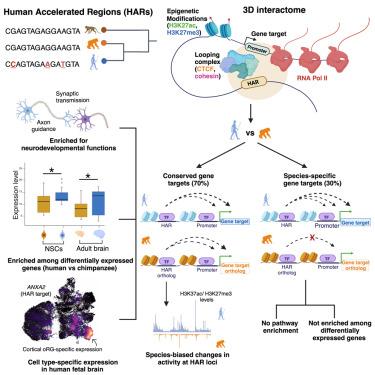
解决人类和黑猩猩神经发育过程中人类加速区域的三维相互作用
人类加速区(HARs)与人类大脑进化有关。然而,缺乏对它们控制的基因和途径的深入了解,阻碍了对它们功能的理解。在这里,我们在人类和黑猩猩神经干细胞(NSCs)中鉴定出1,590个HARs及其同源物的2,963个保守基因靶点。保守的基因靶点丰富于神经发育功能,并且在人类NSCs (hNSCs)和黑猩猩NSCs (cNSCs)以及人类与非人类灵长类动物大脑中鉴定的差异表达基因(deg)中被过度代表。物种特异性的基因靶点不会收敛于任何功能,也不会在deg中富集。HAR靶点在人类胎儿大脑中也表现出细胞类型特异性表达,包括与皮质扩张有关的外放射状胶质细胞。我们的研究结果支持HAR通过改变人类和黑猩猩之间共享的祖先基因靶点的表达而不是通过在人类中获得新的靶点来影响大脑进化,并促进了HAR生物学的假设导向研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


