Defect-Engineered Biomimetic Piezoelectric Nanocomposites With Enhanced ROS Production, Macrophage Re-polarization, and Ca2+ Channel Activation for Therapy of MRSA-Infected Wounds and Osteomyelitis
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
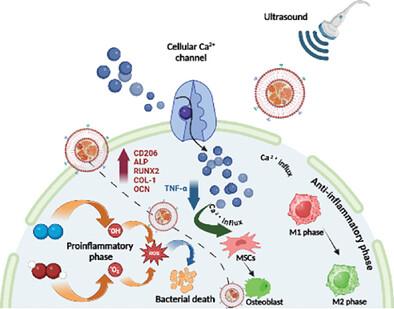
Antibiotic-resistant bacteria often cause lethal infections in both the surficial and deep organs of humans. Failure of antibiotics in resistant infections leads to more effective alternative therapies, like spatiotemporally controllable piezodynamic therapy (PZDT) with deep penetration. Currently, PZDT demands further investigation for improved treatment outcomes and the corresponding therapeutic mechanisms. Herein, a nanocomposite cloaked is reported with a biomimetic coating of TLR-upregulated macrophage membrane for targeted PZDT against MRSA-induced skin wound infection and osteomyelitis, representing surficial and deep infection models, respectively. To boost the therapeutic efficacy, crystal defect engineering is applied by impregnating Fe2+ into bismuth oxy-iodide nanosheets to increase the crystal defects. This results in a significantly higher piezoelectric coefficient than in previous reports, contributing to an amplified reactive oxygen species generation for bacterial killing. More importantly, the notable piezoelectric effect not only re-programs the macrophages into an anti-inflammatory M2 phenotype for accelerating bacterial wound healing but also stimulates the opening of the piezo-stimulated Ca2+ channels and boosts the differentiation of mesenchymal stem cells into osteoblasts for expediting bone tissue repair in osteomyelitis model. Moreover, the Fe-doping supplements T2-magnetic resonance imaging for real-time visualization of nanocomposite distribution. This theranostic system opens a new avenue for future treatment of drug-resistant bacteria-caused diseases.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


