Gelatin-Induced Synthesis of Strain-Engineered Spherical Cu2O Nanoparticles for Efficient Nitrate Reduction to Ammonia
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
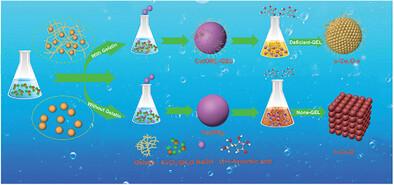
The electrochemical reduction of nitrate to ammonia offers an environmentally sustainable pathway for nitrogen fixation. However, achieving both efficiency and selectivity in nitrate reduction presents a formidable challenge, due to the involvement of sluggish multielectron transfer processes. Herein, the successful synthesis of spherical Cu₂O nanoparticles (s-Cu₂O) exhibiting significant compressive strain effects, achieved through a one-pot method using gelatin as a structural modifier, is reported. The s-Cu₂O catalyst demonstrates exceptional electrochemical performance for nitrate reduction reaction (NO3RR), achieving a Faradaic efficiency (FENH3) of 95.07%, ammonia selectivity of 92.03%, a nitrate conversion rate of 97.77%, and a yield rate of 284.83 µmol h⁻¹ cm⁻2 at −0.8 V versus reversible hydrogen electrode (vs. RHE) for ammonia production. Structural characterization and density functional theory calculations reveal that compressive strain plays a critical role in modulating the electronic structure of the catalyst, thereby activating the *NO intermediate in the potential determining step and effectively suppressing the hydrogen evolution reaction. Furthermore, it is implemented in a Zn-NO3− battery, and the test results indicate that the battery achieved a peak power density of 3.95 mW cm−2 at a potential of 0.129 V (vs Zn/Zn2⁺), illustrating its excellent electrochemical and functional efficacy. This work introduces a novel strategy for the rational design of high-performance electrocatalysts through strain engineering, offering broad implications for energy-efficient ammonia synthesis, and sustainable nitrogen cycling.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


