Enantiomer-Dependent Supramolecular Antibacterial Therapy for Drug-Resistant Bacterial Keratitis
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
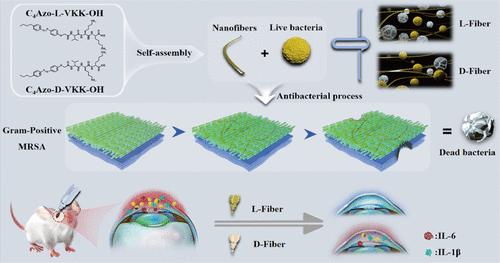
Bacteria have the potential to exhibit divergent stereochemical preferences for different levels of chiral structures, including from molecule, supramolecule, to nanomicroscale helical structure. Accordingly, the structure–activity relationship between chirality and bactericidal activity remains uncertain. In this study, we seek to understand the multivalent molecular chirality effect of chiral supramolecular polymers on antibacterial activity. Two n-butylazobenzene-modified l- and d-tripeptides (abbreviated C4Azo-l-VKK–OH and C4Azo-d-VKK–OH) were synthesized and subsequently self-assembled in water into chiral supramolecular polymers (designated l-Fiber and d-Fiber, respectively). The l-Fiber and d-Fiber displayed comparable nonhelical nanofiber morphologies but exhibited opposite multivalent molecular chirality. A comparative study demonstrated that the l-Fiber exhibited a markedly higher affinity for bacteria, thereby demonstrating significantly enhanced bactericidal efficiency against methicillin-resistant Staphylococcus aureus (MRSA) in comparison to that of the d-Fiber. Following disassembly into monomers via host–guest chemistry, the bactericidal potency of both the l-Fiber and d-Fiber was found to be almost lost, suggesting the multivalent molecular chirality effect. Of note, the l-Fiber exhibited superior efficacy in curing MRSA-infected keratitis in comparison to the d-Fiber. These findings highlight the importance of multivalent molecular chirality in the design and development of chiral supramolecular polymers for antibacterial applications. This research also presents an effective chiral supramolecular antibacterial strategy for the treatment of drug-resistant bacteria-infected keratitis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
文献相关原料
公司名称
产品信息
阿拉丁
0.9% NaCl solution
阿拉丁
HEPES
阿拉丁
Ethanol
阿拉丁
β-Cyclodextrin (β-CD)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


