Continuous Electrochemical Carbon Capture via Redox-Mediated pH Swing─Experimental Performance and Process Modeling
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
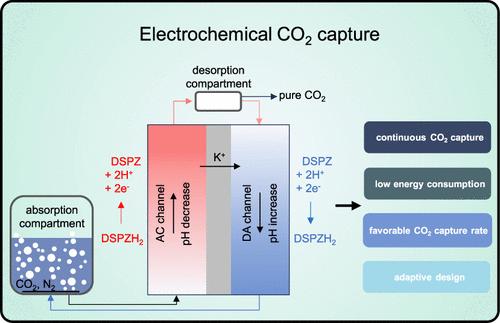
We investigate a continuous electrochemical pH-swing method to capture CO2 from a gas phase. The electrochemical cell consists of a single cation-exchange membrane (CEM) and a recirculation of a mixture of salt and phenazine-based redox-active molecules. In the absorption compartment, this solution is saturated by CO2 from a mixed gas phase at high pH. In the electrochemical cell, pH is reduced, and CO2 is selectively released in a desorption step. We investigate the influence of redox molecule concentration on the charge storage capacity of the solution, as well as the impact of current density and solution recirculation rate on process performance. A theoretical framework, based on a minimal set of assumptions, is established. This framework describes the data very accurately and can be used for system design and optimization. We evaluate the trade-off between energy consumption and CO2 capture rate and compare with published reports. We report a low energy consumption of 32 kJ/mol of CO2 at a capture rate of 39 mmol/m2/min.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


