Combined Carbothermal Reduction–Acetic Acid Leaching Strategy for the Stepwise Recovery of Lithium and Manganese from Spent Electrode Materials
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Carbothermal reduction roasting is an important medium-temperature pyrometallurgical process for efficiently recovering valuable elements from spent lithium-ion batteries (LIBs). In this study, combined carbothermal reduction–acetic acid leaching was proposed, aiming to stepwisely recover Li, Ni, Co, and Mn from spent ternary LIBs containing lithium nickel cobalt manganese oxide (LiNixCoyMnzO2, NCM). The biomass waste materials, chitosan, and chitin were chosen as sustainable reducing agents for carbothermal reduction roasting. The synergistic effect of the reducing gases and biochar produced during pyrolysis led to the reduction of transition metals in NCM into Ni, Co, and MnO, separately. Meanwhile, lithium reacted with CO2 to form Li2CO3. Additionally, an acetic acid leaching process was employed to treat the roasting slag, achieving selective leaching of Li and Mn with leaching efficiencies of 98.45% for Li, 1.20% for Ni, 3.10% for Co, and 98.43% for Mn under optimal conditions. Compared to traditional carbothermal reduction roasting methods, this approach used extracts from shrimp/crab shell solid waste as a source of reducing atmosphere and biochar. More importantly, the leaching behaviors of Li, Ni, Co, and Mn were also investigated. Li and Mn were subsequently recovered as lithium carbonate (Li2CO3) and manganese carbonate (MnCO3), respectively. An economic evaluation was also conducted to present a promising strategy for efficiently recycling valuable metals from spent LIBs.
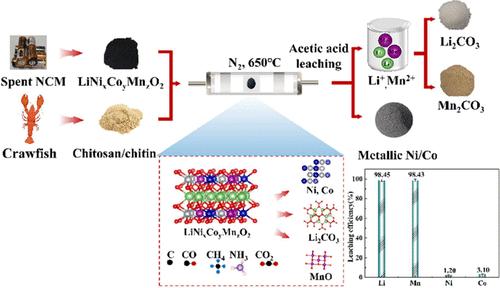
碳热还原-醋酸联合浸出从废电极材料中逐步回收锂和锰
碳热还原焙烧是高效回收废锂离子电池中有价元素的重要中温热法工艺。本研究提出了碳热还原-乙酸联合浸出的方法,旨在从含锂镍钴锰氧化物(LiNixCoyMnzO2, NCM)的废三元锂中分步回收Li、Ni、Co和Mn。选择生物质废弃物、壳聚糖和几丁质作为可持续还原剂进行碳热还原焙烧。热解过程中产生的还原性气体和生物炭的协同作用导致NCM中的过渡金属分别还原为Ni、Co和MnO。同时,锂与CO2反应生成Li2CO3。采用乙酸浸出工艺对焙烧渣进行处理,在最佳浸出条件下,Li、Ni、Co、Mn的浸出率分别为98.45%、1.20%、3.10%、98.43%,实现了Li、Mn的选择性浸出。与传统的碳热还原焙烧方法相比,该方法使用虾/蟹壳固体废物提取物作为还原性大气和生物炭的来源。更重要的是,还研究了Li、Ni、Co和Mn的浸出行为。Li和Mn分别被回收为碳酸锂(Li2CO3)和碳酸锰(MnCO3)。还进行了经济评估,以提出有效地从废lib中回收有价金属的有前途的战略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


