Preparation of a Cu–Ce–O-Modified Activated Carbon Adsorbent to Enhance Hydrogen Sulfide Removal at Low Temperatures
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
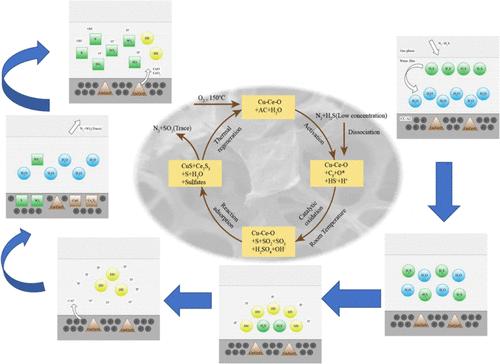
It is necessary to remove trace amounts of hydrogen sulfide (H2S) to a low degree at low temperatures; otherwise, it will lead to an increase in industrial desulfurization difficulty. Nano–Cu–Ce oxide-modified activated carbon adsorbents with different active components are synthesized by the sol–gel method and ball milling method. The adsorption properties of the materials are tested by the fixed-bed adsorbent evaluation system. The results show that 15%CuCe0.75Ox/activated carbon (CAC-15) has the best adsorption performance for H2S, reaching 103.58 mg/g in an O2-free ambience under 40 °C. The composite had high adsorption capacity for H2S, and its adsorption property decreases with the increase of space velocity and H2S concentration and fluctuates with the increase of temperature. At 60 °C, the maximum absorption capacity is 155.59 mg/g. The desulphurization process includes active adsorption and catalytic oxidation, and the adsorption capacity is determined by the chemical property and surface structure of the active component. The adsorbent has a highly mesoporous structure, which was highly dependent on the loading of the Cu–Ce–O active component. The results of chemical characterization show that sulfur was the main adsorption product, and strong alkaline sites play a major role. The mechanism of H2S adsorption by the adsorbent is described. This study provides new insights into the Cu/Ce-doped activated carbon adsorbent and the reactive adsorption of H2S.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


