Design, Synthesis and Anti-Inflammatory Evaluation of 3-Substituted 5-Amidobenzoate Derivatives as Novel P2Y14 Receptor Antagonists via Structure-Guided Molecular Hybridization
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
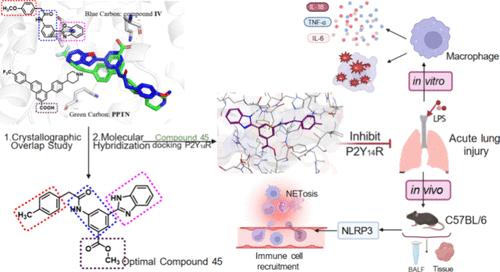
The P2Y14R is activated by UDP and UDP glucose and is involved in many human inflammatory diseases. Based on the molecular docking analysis of currently reported P2Y14R antagonists and the crystallographic overlap study between PPTN and compound IV, a series of 3-substituted 5-amidobenzoate derivatives were designed, synthesized, and identified as promising P2Y14R antagonists. The optimal compound 45 (methyl 3-(1H-benzo[d]imidazol-2-yl)-5-(2-(p-tolyl) acetamido)benzoate, IC50 = 0.70 ± 0.01 nM) showed a strong binding ability to P2Y14R, high selectivity, moderate oral bioactivity, and improved pharmacokinetic profiles. In the LPS-induced acute lung injury model, compound 45 demonstrated significant anti-inflammatory efficacy, effectively mitigating the pulmonary infiltration of immune cells and inflammatory response through suppressing the NLRP3 signaling pathway. Thus, 45 with potent P2Y14R antagonistic activity, in vitro and vivo efficacy, and favorable druggability can be a strategy for treating acute lung injury and can be optimized in further studies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


