Low-Level Fe Doping in CoMoO4 Enhances Surface Reconstruction and Electronic Modulation Creating an Outstanding OER Electrocatalyst for Water Splitting
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Efficient and stable nonprecious metal-based oxygen evolution reaction (OER) electrocatalysts are pivotal for water electrolysis technology. Herein, we are reporting an effective strategy for fabricating efficient Co-based OER electrocatalysts by low-level Fe doping in CoMoO4 to boost surface reconstruction and electronic modulation, which resulted in excellent OER electroactivity consequently. Our findings reveal that a mere 5.30% (wt %) of Fe doping can raise the O 2p band center energy nearer to the Fermi level, reduce the energy barrier for oxygen vacancy (VO) formation, and significantly enhance OER electrocatalytic activity. Additionally, the fast dissolution of Mo in the initial reaction facilitated surface reconstruction to form active oxyhydroxide species and stabilized Fe and Co from leaching. As a result, the optimized catalyst Fe-CoMoO4-0.2 exhibited a low overpotential of 276 mV at 10 mA cm–2 in 1 M KOH and can operate stably at a current density of 20 mA cm–2 for at least 24 h under water splitting conditions. This work provides an excellent example of regulating the surface structure and electronic properties of the OER catalysts.
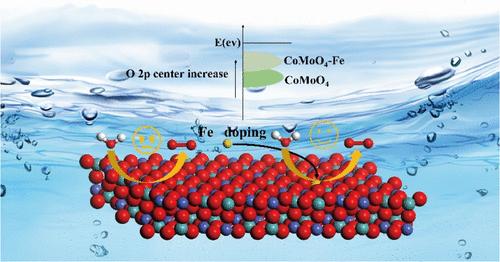
在CoMoO4中掺杂低水平铁增强了表面重构和电子调制,创造了一种出色的OER水分解电催化剂
高效、稳定的非贵金属析氧反应电催化剂是水电解技术的关键。在此,我们报告了一种有效的策略,通过在CoMoO4中掺杂低水平的铁来制造高效的co基OER电催化剂,以促进表面重构和电子调制,从而产生优异的OER电活性。我们的研究结果表明,仅5.30% (wt %)的Fe掺杂就能使o2p带中心能提高到接近费米能级,降低氧空位(VO)形成的能垒,并显著提高OER电催化活性。此外,Mo在初始反应中的快速溶解促进了表面重构,形成活性的氢氧化物,并稳定了Fe和Co的浸出。结果表明,优化后的Fe-CoMoO4-0.2催化剂在1 M KOH条件下,在10 mA cm-2下具有较低的过电位276 mV,在20 mA cm-2电流密度下可稳定工作至少24 h。这项工作为调节OER催化剂的表面结构和电子性能提供了一个很好的例子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


