Functionalization of Indoles with 1,3,5-Triazinanes: Chemistry of Aminomethylation vs the Hofmann–Martius-Type Rearrangement
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
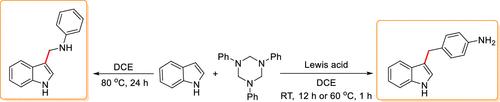
We have developed efficacious routes toward the selective synthesis of two classes of compounds such as C-3 amino-methylated indoles and 4-indol-3-yl-methylanilines from the same precursors, namely, indoles and 1,3,5-triazinanes. It is reported that the controlled cleavage of 1,3,5-triazinanes can be effected by heat for the generation of aryl imine motifs, and we observed that the presence of Lewis acid influences the course of these transformations toward different products. The reaction toward indol-3-yl-methylanilines proceeds via a nucleophilic attack of indole to the aryl imine generated from the 1,3,5-triazinanes to form an amino-methylated product which undergoes a Lewis acid mediated Hofmann–Martius-type rearrangement. In the absence of a Lewis acid, the reaction between indoles and 1,3,5-triazinane afforded C-3 amino-methylated indoles. Experimentally, we could prove that the amino-methylated product was the intermediate formed during the Lewis acid catalyzed synthesis of 4-indol-3-yl-methylanilines and that the process proceeds in an intermolecular fashion. The selective synthesis of both classes of compounds was found to be general, and a library of molecules was generated.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


