Crystal Phase-Dependent Dispersion and Catalysis of the Ag Species Supported on TiO2 for CO Oxidation with Excess Oxygen
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Oxidation-induced dispersion of supported metal catalysts has been frequently observed in gas–solid heterogeneous reactions, while precise tailoring of the structures of restructured metals remains challenging. Here, we successfully demonstrated the feasibility of using different TiO2 crystal phases to tune the nanostructures of restructured silver species upon CO oxidation with excess O2. Compared to pure anatase and rutile phases, a mixture of anatase and rutile phases (m-TiO2) is more advantageous for the dispersion of supported Ag species, with a particle size distribution of 3.5 ± 0.2 nm, which is closely related to the surface OH group and defect concentrations of TiO2 supports. Spectroscopic characterizations clearly reveal the CO oxidation catalyzed by the Ag/TiO2 catalysts following a Mars–van Krevelen mechanism. Consequently, in addition to the Ag dispersion, a Ag/m-TiO2 catalyst with higher active oxygen species contents and correspondingly better reducibility, relevant for CO activation and reactivity, contributes to better catalytic performance in CO oxidation. These results highlight the potential of crystal phases of oxide supports in tailoring oxidation-induced restructuring to develop efficient heterogeneous catalysts for applications.
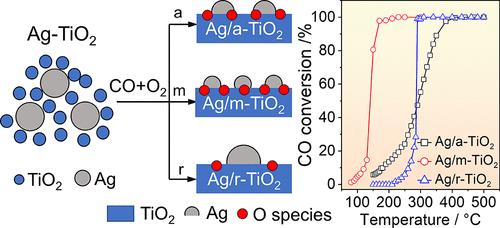
在过量氧气的 CO 氧化过程中,TiO2 上支持的 Ag 物种的晶相依赖性分散和催化作用
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


