Tertiary Alcohols as Mechanistic Probes for Photocatalysis: the Gas-Phase Reaction of 2-Methyl-2-Pentanol on Titania P25 in a Microphotoreactor
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Despite intense research in heterogeneous photocatalysis, a lack of mechanistic understanding still hinders the rational design of efficient photocatalysts to make them competitive with thermal processes that currently dominate the industry. This study elucidates the underlying mechanism of photoreactions by employing tertiary alcohols as probe molecules on a titania P25 catalyst for the understanding of photocatalytic reactions on a molecular scale. We show that the reactions do not follow the commonly assumed reaction mechanism of separate but coupled redox reactions. Instead, the gas-phase reaction occurs selectively via a homolytic bond cleavage of the long alkyl chain, leading to the formation of the corresponding ketone and an alkane, as exemplified for 2-methyl-2-pentanol at ambient pressure. The alkane stems predominantly from the recombination of the alkyl-moiety with surface hydrogen. Additionally, we demonstrate that the alkyl moiety can also undergo a dimerization reaction forming a long chain alkane, which is facilitated on bare TiO2. The high time-resolution enabled by the used microreactor allowed us to confirm that this side reaction is a higher-order process, which is governed by the alcohol surface coverage on TiO2. The parallels of the observed reaction properties with studies performed on a TiO2(110) single crystal in vacuum reveal that no significant pressure and material gap exists. On the one hand, this strongly suggests that also the reaction mechanism for the conversion of other alcohols must be reconsidered on titania-based photocatalysts and, on the other hand, demonstrates the potential of tertiary alcohols as mechanistic probes in photocatalysis. Moreover, the highly selective reactions of tertiary alcohols may open up alternative routes for chemical synthesis.
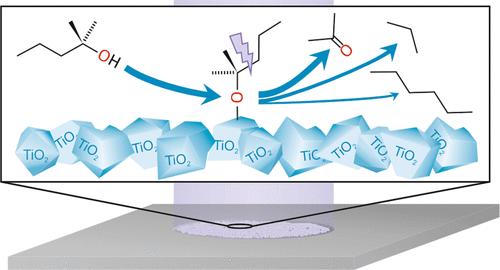
作为光催化机理探针的叔醇:微光反应器中 2-甲基-2-戊醇在二氧化钛 P25 上的气相反应
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


