Spin-state regulation of heteronuclear Cu-Co dual-atomic sites via tuning electronic asymmetry for enhanced oxygen reduction
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
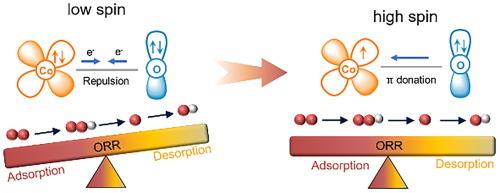
Modulating electronic asymmetry of transition metal (TM) sites attributes to switching their spin state and regulating bonding to oxygen-containing intermediates, thereby facilitating their performance for oxygen reduction reaction (ORR). Herein, we engineer the local coordination structure of the Cu-Co dual-atomic site by integrating phosphorous. The constructed CuCoNPC therefore possesses asymmetric active sites (CoN3-CuN3P), leading to a high spin-state of reactive Co sites. The accordingly tuned dyz orbital occupation consequently triggers the rate-determining step of ORR switching from first (*O2→*OOH) to the last protonation (*OH → H2O). As a result, the CuCoNPC exhibits exceptional ORR activity, with jk of 54 mA cm−2 at 0.75 VRHE and half-wave potential (E1/2) of 0.86 VRHE, overwhelming that of Pt in alkaline electrolyte. Meanwhile, it also displays a Pt-comparable onset (0.82 VRHE) and E1/2 (0.72 VRHE) in acidic media. Finally, the CuCoNPC catalyst superior performance in liquid-form (Pmax = 194 mW cm−2) and all-solid-state flexible (OCP = 1.51 V) zinc-air batteries. This work provides valuable guidance in developing active TM-based ORR catalysts via tuning electronic asymmetry.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


