Identification, structure, and agonist design of an androgen membrane receptor
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
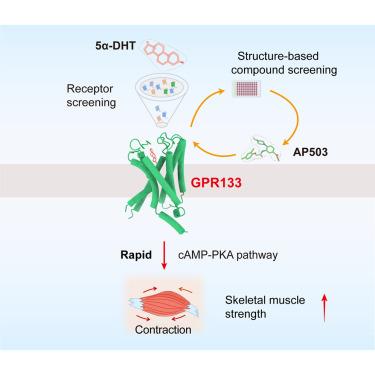
Androgens, such as 5α-dihydrotestosterone (5α-DHT), regulate numerous functions by binding to nuclear androgen receptors (ARs) and potential unknown membrane receptors. Here, we report that the androgen 5α-DHT activates membrane receptor GPR133 in muscle cells, thereby increasing intracellular cyclic AMP (cAMP) levels and enhancing muscle strength. Further cryoelectron microscopy (cryo-EM) structural analysis of GPR133-Gs in complex with 5α-DHT or its derivative methenolone (MET) reveals the structural basis for androgen recognition. Notably, the presence of the “Φ(F/L)2.64-F3.40-W6.53” and the “F7.42××N/D7.46” motifs, which recognize the hydrophobic steroid core and polar groups, respectively, are common in adhesion GPCRs (aGPCRs), suggesting that many aGPCRs may recognize different steroid hormones. Finally, we exploited in silico screening methods to identify a small molecule, AP503, which activates GPR133 and separates the beneficial muscle-strengthening effects from side effects mediated by AR. Thus, GPR133 represents an androgen membrane receptor that contributes to normal androgen physiology and has important therapeutic potentials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
文献相关原料
公司名称
产品信息
索莱宝
Eosin
索莱宝
Hematoxylin
阿拉丁
Benzamidine hydrochloride
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


