Signature cytokine-associated transcriptome analysis of effector γδ T cells identifies subset-specific regulators of peripheral activation
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
γδ T cells producing either interleukin-17A (γδ17 cells) or interferon-γ (γδIFN cells) are generated in the mouse thymus, but the molecular regulators of their peripheral functions are not fully characterized. Here we established an Il17a-GFP:Ifng-YFP double-reporter mouse strain to analyze at unprecedented depth the transcriptomes of pure γδ17 cell versus γδIFN cell populations from peripheral lymph nodes. Within a very high fraction of differentially expressed genes, we identify a panel of 20 new signature genes in steady-state γδ17 cells versus γδIFN cells, which we further validate in models of experimental autoimmune encephalomyelitis and cerebral malaria, respectively. Among the signature genes, we show that the co-receptor CD6 and the signaling protein Themis promote the activation and proliferation of peripheral γδIFN cells in response to T cell antigen receptor stimulation in vitro and to Plasmodium infection in vivo. This resource can help to understand the distinct activities of effector γδ T cell subsets in pathophysiology. Here, the authors use a double-reporter system to tag IFNγ-producing versus IL-17A-producing γδ T cells to compile a trancriptomic resource of these cell subsets in mice at steady state and in response to cerebral malaria or multiple sclerosis.

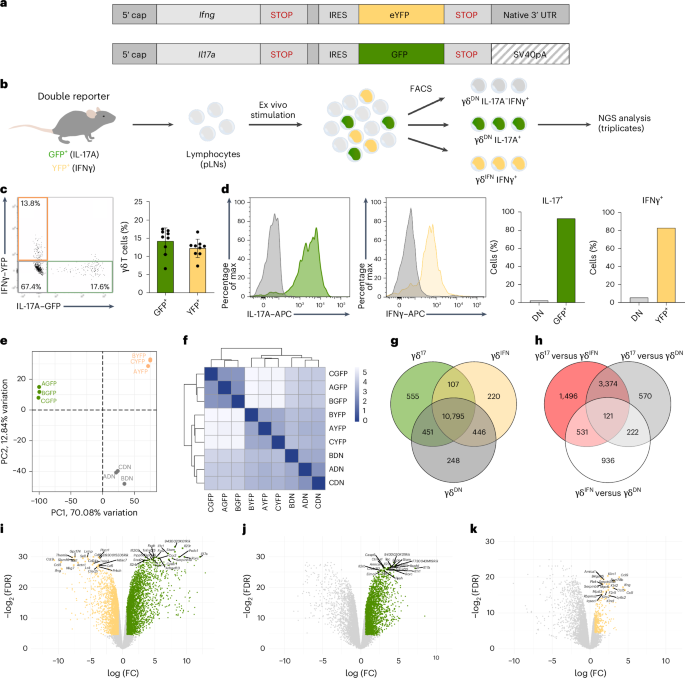
效应γδ T细胞的特征细胞因子相关转录组分析鉴定了外周活化的亚群特异性调节因子
小鼠胸腺中产生产生白细胞介素- 17a (γδ17细胞)或干扰素-γ (γδ ifn细胞)的γδ T细胞,但其外周功能的分子调节因子尚未完全表征。在这里,我们建立了Il17a-GFP:Ifng-YFP双报告小鼠菌株,以前所未有的深度分析来自外周淋巴结的纯γδ17细胞和γδIFN细胞群的转录组。在非常高比例的差异表达基因中,我们在稳态γδ17细胞和γδIFN细胞中鉴定了一组20个新的特征基因,我们分别在实验性自身免疫性脑脊髓炎和脑疟疾模型中进一步验证了这一结果。在这些特征基因中,我们发现共受体CD6和信号蛋白Themis促进外周γδIFN细胞在体外响应T细胞抗原受体刺激和体内响应疟原虫感染时的激活和增殖。这一资源有助于了解在病理生理中γδ T细胞亚群的不同活动。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


