Safety and efficacy of selective RIPK1 inhibitor SIR1-365 in hospitalized patients with severe COVID-19: A multicenter, randomized, double-blind, phase 1b trial
引用次数: 0
Abstract
Background
Receptor-interacting protein kinase 1 (RIPK1), a serine/threonine protein kinase, is mainly activated by pro-inflammatory cytokines and pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its activation could result in apoptosis, necroptosis, or inflammation. This study was conducted to evaluate the safety and efficacy of a potent and selective inhibitor of RIPK1, SIR1-365, in hospitalized patients with severe coronavirus disease 2019 (COVID-19).
Methods
This multicenter, randomized, double-blind, phase 1b study screened patients from December 18, 2020 until November 27, 2021. Adults hospitalized with severe COVID-19 (diagnosed ≤2 weeks before screening) were randomized 1:1 to receive oral placebo or SIR1-365 100 mg three times daily for ≤14 consecutive days, with standard-of-care. The primary objective was to evaluate SIR1-365 safety and tolerability. Secondary objectives included an assessment of SIR1-365 efficacy. Descriptive statistics were used to summarize safety. The study was not powered for efficacy testing. Relevant inferential statistical tests were used to aid interpretation of differences in clinical efficacy.
Results
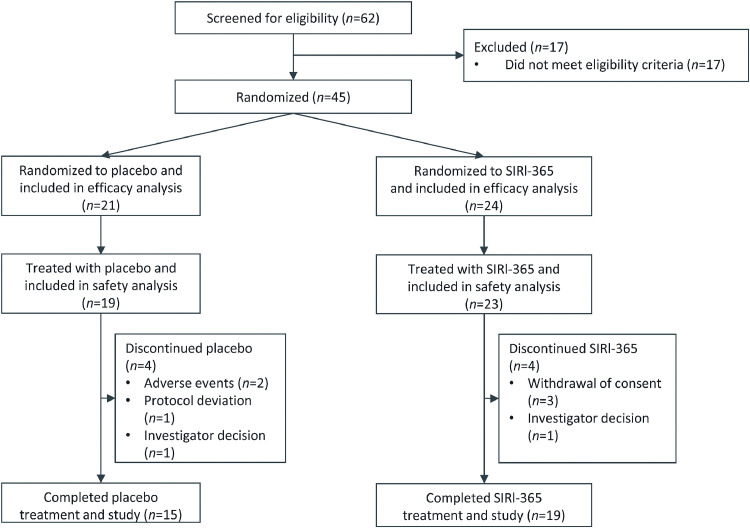
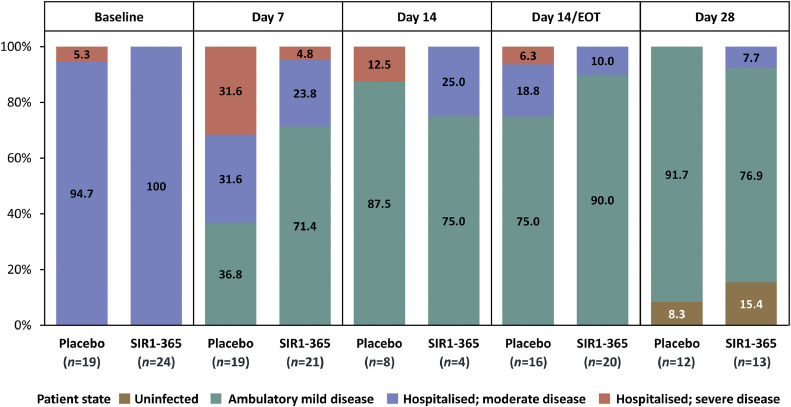
Forty-five patients were randomized, 42 were treated. Eighteen patients experienced treatment-emergent adverse events (TEAEs) and 7 patients were ≥ grade 3. Fewer SIR1-365-treated vs. placebo-treated patients experienced TEAEs (30.4% vs. 57.9%) and serious TEAEs (13.0% vs. 26.3%) within 28 days of the first dose. There were no serious treatment-related TEAEs or deaths. Compare to placebo, SIR1-365 significantly increased arterial oxygenation from baseline to day 7 (least-squares mean change [standard error]: 109.4 [26.4] vs. -24.2 [23.6]; P=0.0095), significantly reduced hospitalization duration after treatment (mean±standard deviation: [4.7±3.7] days vs. [8.6±5.6] days; P=0.0145) and respiratory failure incidence (8.3% vs. 38.1%; two-sided P=0.0291) during the study, and numerically shortened the time to clinical improvement in World Health Organization ordinal scale (median: 5.0 days vs. 9.0 days, P=0.0766).
Conclusions
SIR1-365 was well tolerated and demonstrated a trend toward quicker recovery than placebo in hospitalized patients with severe COVID-19.
Trial Registration ClinicalTrials.gov number: NCT04622332
选择性RIPK1抑制剂SIR1-365在重症COVID-19住院患者中的安全性和有效性:一项多中心、随机、双盲、1b期试验
背景:受体相互作用蛋白激酶1 (Receptor-interacting protein kinase 1, RIPK1)是一种丝氨酸/苏氨酸蛋白激酶,主要被促炎细胞因子和病原体激活,包括严重急性呼吸综合征冠状病毒2 (SARS-CoV-2),其激活可导致细胞凋亡、坏死或炎症。本研究旨在评估RIPK1强效选择性抑制剂SIR1-365在2019年严重冠状病毒病(COVID-19)住院患者中的安全性和有效性。方法:这项多中心、随机、双盲、1b期研究从2020年12月18日至2021年11月27日筛查患者。因严重COVID-19住院的成人(筛查前诊断≤2周)按1:1随机分组,接受口服安慰剂或SIR1-365 100 mg,每日3次,连续≤14天。主要目的是评估SIR1-365的安全性和耐受性。次要目标包括SIR1-365的疗效评估。采用描述性统计对安全性进行总结。这项研究没有功效测试。使用相关的统计检验来解释临床疗效的差异。结果:随机分组45例,治疗42例。18例患者出现治疗不良事件(teae), 7例患者≥3级。sir1 -365治疗组与安慰剂治疗组相比,在首次给药后28天内出现teae (30.4% vs 57.9%)和严重teae (13.0% vs 26.3%)的患者较少。没有与治疗相关的严重teae或死亡。与安慰剂相比,SIR1-365从基线到第7天显著增加动脉氧合(最小二乘平均变化[标准误差]:109.4[26.4]对-24.2 [23.6];P=0.0095),治疗后住院时间显著缩短(平均±标准差:[4.7±3.7]天vs.[8.6±5.6]天;P=0.0145)和呼吸衰竭发生率(8.3% vs. 38.1%;双侧P=0.0291),并在数值上缩短了世界卫生组织(World Health Organization)顺序量表的临床改善时间(中位数:5.0天vs. 9.0天,P=0.0766)。结论:SIR1-365耐受性良好,在重症COVID-19住院患者中显示出比安慰剂更快恢复的趋势。临床试验注册。gov号码:NCT04622332。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of intensive medicine
Critical Care and Intensive Care Medicine
CiteScore
1.90
自引率
0.00%
发文量
0
审稿时长
58 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


