Intestine-derived fibroblast growth factor 19 alleviates lipopolysaccharide-induced liver injury by regulating bile acid homeostasis and directly improving oxidative stress
引用次数: 0
Abstract
Background
Cholestasis plays a critical role in sepsis-associated liver injury (SALI). Intestine-derived fibroblast growth factor 19 (FGF19) is a key regulator for bile acid homeostasis. However, the roles and underlying mechanisms of FGF19 in SALI are still unclear.
Methods
We conducted a case–control study that included 58 pediatric patients aged from 1 month to 14-years-old diagnosed with sepsis at Shanghai Children's Hospital from January to December 2018 and 30 healthy individuals. The serum FGF19 levels of these patients with sepsis were analyzed and compared with those of healthy controls. Recombinant human FGF19 was intravenously injected in mice once a day for 7 days at a dose of 0.1 mg/kg body weight before lipopolysaccharide (LPS) treatment. Liver bile acid profiles and the gene expression involved in bile acid homeostasis were investigated in the mice groups. Metabolomic data were further integrated and analyzed using Ingenuity Pathways Analysis (IPA) software. In the in vitro analysis using HepG2 cells, the influence of FGF19 pretreatment on reactive oxygen species (ROS) production and mitochondrial dysfunction was analyzed. Compound C (CC), an inhibitor of AMP-activated protein kinase (AMPK) activation, was used to confirm the roles of AMPK activation in FGF19-mediated hepatoprotective effects.
Results
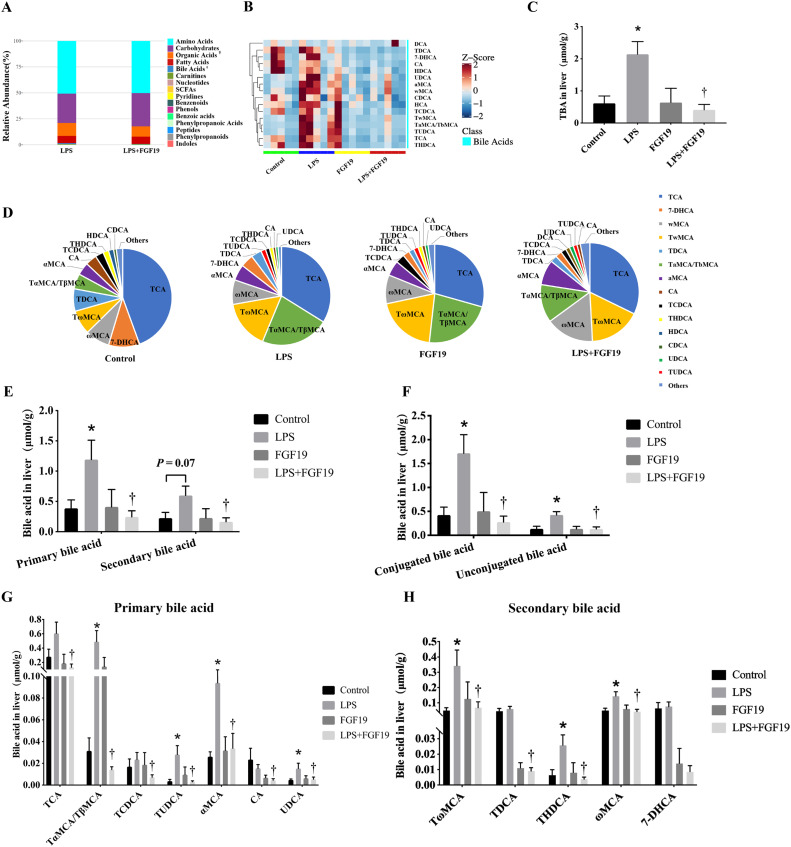
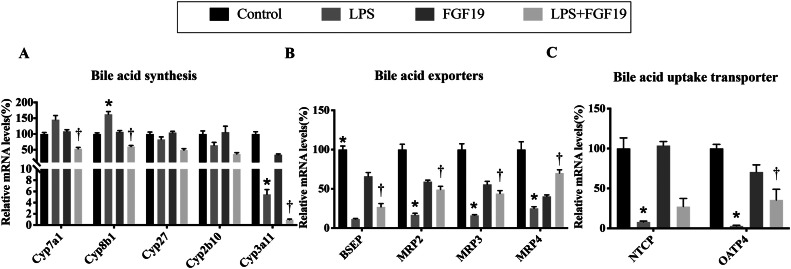
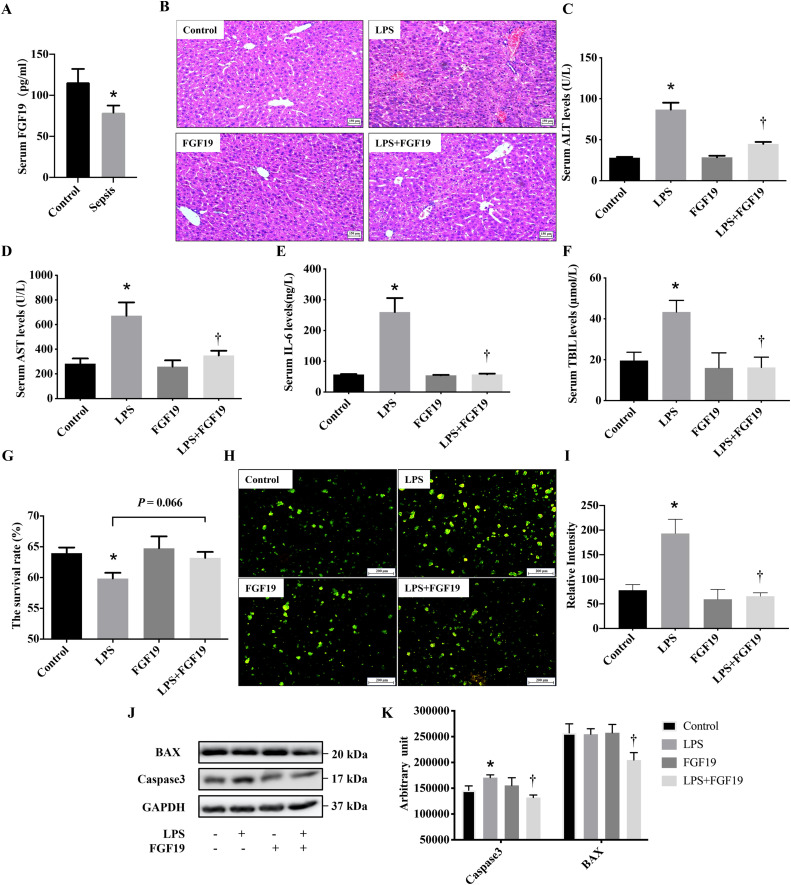
Serum FGF19 levels were significantly lower in children with sepsis than in healthy controls (115 pg/mL vs. 79 pg/mL, P=0.03). Pre-administration of recombinant human FGF19 alleviated LPS-induced acute liver injury (ALI) and improved LPS-induced cholestasis in mice. Moreover, FGF19 directly reversed LPS-induced intracellular ROS generation and LPS-decreased mitochondrial membrane potential in vitro and in vivo, resulting in hepatoprotection against LPS-induced apoptosis. More importantly, the inhibition of AMPK activity partially blocked the protective effects of FGF19 against LPS-induced oxidative stress and mitochondrial dysfunction.
Conclusions
Intestine-derived FGF19 alleviates LPS-induced ALI via improving bile acid homeostasis and directly suppressing ROS production via activating the AMPK signaling pathway.
肠源性成纤维细胞生长因子19通过调节胆汁酸稳态和直接改善氧化应激来减轻脂多糖诱导的肝损伤。
背景:胆汁淤积在脓毒症相关性肝损伤(SALI)中起关键作用。肠源性成纤维细胞生长因子19 (FGF19)是胆汁酸稳态的关键调节因子。然而,FGF19在SALI中的作用和潜在机制尚不清楚。方法:我们对2018年1月至12月在上海儿童医院诊断为败血症的58例1个月至14岁的儿童患者和30名健康个体进行了病例对照研究。分析这些败血症患者的血清FGF19水平,并与健康对照进行比较。在脂多糖(LPS)处理前,以0.1 mg/kg体重的剂量静脉注射重组人FGF19,每天1次,连续7天。研究各组小鼠肝脏胆汁酸谱及胆汁酸稳态相关基因表达。代谢组学数据进一步整合并使用Ingenuity Pathways Analysis (IPA)软件进行分析。在HepG2细胞的体外分析中,分析了FGF19预处理对活性氧(ROS)产生和线粒体功能障碍的影响。化合物C (CC)是一种amp活化蛋白激酶(AMPK)活化抑制剂,用于证实AMPK活化在fgf19介导的肝保护作用中的作用。结果:脓毒症患儿血清FGF19水平显著低于健康对照组(115 pg/mL vs 79 pg/mL, P=0.03)。重组人FGF19预给药可减轻lps诱导的小鼠急性肝损伤(ALI),改善lps诱导的小鼠胆汁淤积。此外,在体外和体内,FGF19直接逆转lps诱导的细胞内ROS生成和lps降低的线粒体膜电位,从而对lps诱导的细胞凋亡产生肝脏保护作用。更重要的是,AMPK活性的抑制部分阻断了FGF19对lps诱导的氧化应激和线粒体功能障碍的保护作用。结论:肠源性FGF19通过改善胆汁酸稳态和激活AMPK信号通路直接抑制ROS的产生来缓解lps诱导的ALI。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of intensive medicine
Critical Care and Intensive Care Medicine
CiteScore
1.90
自引率
0.00%
发文量
0
审稿时长
58 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


