Metabolic Fingerprint of Dual Body Fluids Deciphers Diabetic Retinopathy
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
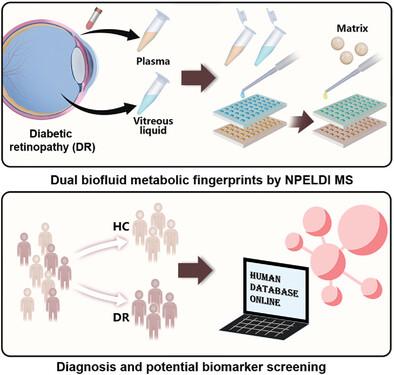
Diabetic retinopathy (DR) is a microvascular complication of diabetes, affecting 34.6% of diabetes patients worldwide. Early detection and timely treatment can effectively improve the prognosis of DR. Metabolomic analysis provides a powerful tool for studying pathophysiological processes. Conducting metabolomic analyses on DR-related biofluids helps identify differential metabolic expressions during disease progression, thereby discovering potential biomarkers to support clinical diagnosis and treatment. Here, an innovative workflow for vitreous liquid analysis is established, and a machine learning-based DR analysis platform integrating vitreous liquid metabolic fingerprint (VL-MF) and plasma metabolic fingerprint (P-MF) derived via nanoparticle enhanced laser desorption/ionization mass spectrometry is developed. Direct VL-MF and P-MF are obtained with desirable reproducibility (coefficient of variation, CV <5%) and remarkable speed (3 s per sample), and DR patients are distinguished from healthy controls applying dual biofluid-MF with an area under the curve (AUC) of 0.957. Moreover, a biomarker candidate panel from vitreous liquid and plasma with an AUC of 0.945 is constructed and the related metabolic pathways are identified by metabolomics pathway analysis (MetPA). This work offers a powerful multi-biofluid platform that can not only contribute to DR but also provide solid references for other clinical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


