Improving the Electrochemical Properties of SiOx Anode for High-Performance Lithium-Ion Batteries by Magnesiothermic Reduction and Prelithiation
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
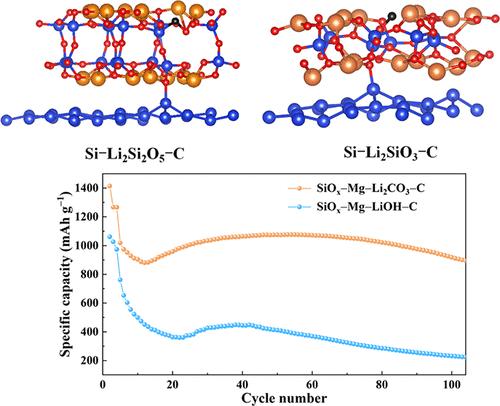
For lithium-ion batteries, silicon monoxide is a potential anode material, but its application is limited by its relatively large irreversible capacity loss, which leads to its low initial Coulombic efficiency (ICE). In this study, we conduct a two-step reaction for the formation of silicon oxide-based materials, including a magnesiothermic reduction of SiOx with Mg, followed by the solid-state lithiation of silicon oxide with Li2CO3. Our results demonstrate that Mg can reduce SiO2 to Si and form MgSiO3, while Li2CO3 reacts with SiOx to form Li2Si2O5. MgSiO3 and Li2Si2O5 on the surface of SiOx can effectively mitigate the irreversible loss of lithium ions, thus enhancing the ICE of SiOx. The resulting SiOx–Mg–Li2CO3–C nanostructure has an ICE of up to 91.1% and a relatively stable cycle performance. After 100 cycles at 0.5 C, the capacity is still 894.5 mAh g–1, and the capacity retention rate is 87.9%. A lithium-ion full battery with the commercial LiNi0.8Mn0.1Co0.1O2 (NCM811) as the cathode was assembled to test its practical applicability. The full cell exhibits a stable discharge capacity of 91.4 mAh g–1 after 100 cycles at 1 C, with a capacity retention of 79.9%.
通过镁热还原和预硅化改善高性能锂离子电池用氧化硅负极的电化学特性
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


