Boosting electrocatalytic nitrate reduction to ammonia with a Cu/Ag-Ru tandem catalyst at industrial-scale current density
IF 10.7
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The nitrate reduction reaction (NO3RR) represents a promising route for water treatment and NH3 generation. This process involves the deoxygenation of NO3− to form nitrite (NO2−), followed by its subsequent hydrogenation. However, discrepancies in the rates of these two steps result in a decrease in faradaic efficiency (FE) and NH3 yield rate. Herein, we demonstrated a tandem catalyst of (Cu7/Ag3)7-Ru3/C achieving a high NH3 yield rate of 3.45 mmol h−1 cm−2 (2.30 mol gcat−1 h−1) and a FE of 93.48% at −0.9 V vs. the reversible hydrogen electrode. The Cu/Ag heterostructure greatly enhanced the conversion of NO3− to NO2− over a wide potential window due to the synergistic effect, while Ru, selectively adsorbing NO2−, provided active hydrogen derived from water hydrolysis to facilitate NH3 synthesis. Furthermore, (Cu7/Ag3)7-Ru3/C exhibited stable performance in a membrane electrode assembly over 60 hours, achieving an average NH3 yield rate of 6.90 mmol h−1. The ammonium chloride solid product was successfully obtained using an air stripping methodology. In situ characterization revealed that the surface microenvironment of Ru influenced the adsorption configuration of *NO and on-top adsorbed NO was more favorable for ammonia synthesis compared to bridge-adsorbed NO. The overall reaction pathway involved stepwise deoxygenation to form *N and subsequent gradual hydrogenation.
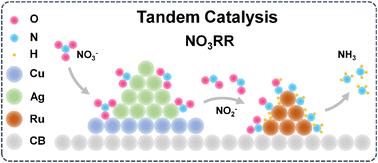
在工业级电流密度下使用铜/银-铜串联催化剂促进电催化硝酸盐还原为氨气
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


