An efficient catalytic route in haem peroxygenases mediated by O2/small-molecule reductant pairs for sustainable applications
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
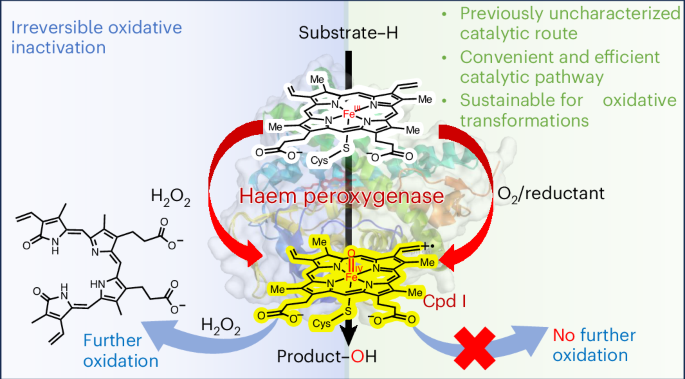
Haem peroxygenases are attractive biocatalysts for incorporating oxygen into organic molecules using H2O2. However, their practical applications are hindered by irreversible oxidative inactivation due to exogenous H2O2 usage. Here we present an alternative catalytic route in haem peroxygenases that uses O2 and small-molecule reductants such as ascorbic acid and dehydroascorbic acid (DHA) to drive reactions. Our experimental and computational studies indicated that DHAA, the hydrated form of DHA, serves as the key co-substrate that activates oxygen to generate the active oxyferryl haem compound I. We also demonstrate the broad applicability of this O2/reductant-dependent route across various haem peroxygenases, highlighting its biological significance for mono-oxygenase functionality. Importantly, this innovative route avoids the use of H2O2, thereby preventing the risk of irreversible enzyme inactivation. Finally, scaled-up reactions yielded chiral, value-added products with excellent productivity, underscoring the synthetic potential of this developed peroxygenase technology for sustainable chemical transformations. H2O2-dependent haem-peroxygenase-catalysed C–H bond oxyfunctionalization reactions have attracted much attention, but elevated concentrations of H2O2 are detrimental to the enzyme. Now, it is reported that this biocatalyst can operate via an alternative pathway using O2 and small-molecule reductants.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


