Unraveling the Mechanism and Influence of Auxiliary Ligands on the Isomerization of Neutral [P,O]-Chelated Nickel Complexes for Olefin Polymerization
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
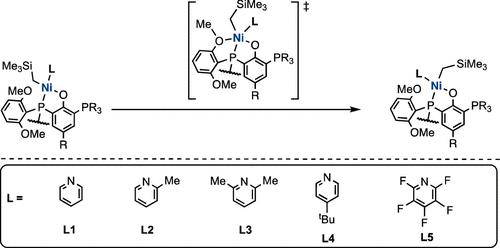
The copolymerization of ethylene with polar monomers presents a significant challenge. While palladium catalysts have shown promise, nickel catalysts are more economical but suffer from poor activity. Previous studies suggest that the isomerization step involved in the nickel-catalyzed polymerization may influence the catalyst activities. Herein, we explore the isomerization mechanisms of two phosphine-phenoxide-ligated catalysts using density functional theory (DFT) studies. We found that out of dissociative, tetrahedral, and associative mechanisms, the associative mechanism is the likeliest, with a pendant methoxy oxygen atom from the ligand to fulfill the fifth coordination site on nickel before Berry pseudorotation. The effect of varying auxiliary ligands on the activation barrier heights was also investigated and found that electron-releasing alkyl groups on substituted pyridine ligands have diminished electronic influence on pseudorotational barriers, but if present at the ortho-positions, will elevate the barriers due to larger steric influences. The electron-withdrawing groups on the ligand result in weaker ligand binding and lower pseudorotational barriers. These insights into the mechanisms of cis-trans isomerization and auxiliary ligand effects may offer valuable guidance for optimizing catalyst performance in copolymerization processes by lowering the barrier of isomerization by fine-tuning the steric and electronic influences of auxiliary ligands and enhancing overall copolymerization efficiency.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


