Multifactorial Analysis of the Collapse Process of Double Bubbles Based on the Coarse-Grained Force Field in Free Domain
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
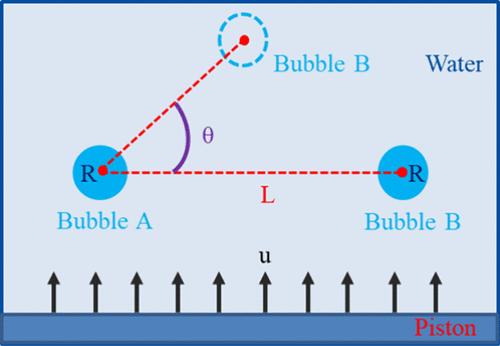
Cavitation has been a hot research topic for scholars in various fields because of the intense mechanical, chemical, and thermal effects of bubble collapse. It forms a cluster of bubbles, and the bubbles will affect, interfere with, and couple with each other. To grasp the main factors affecting bubble collapse and the interbubble mechanism, the paper adopts the molecular dynamics simulation combined with the coarse-grained force field to study the collapse process of the double bubble model and takes the dynamic shape change of the bubbles, the local velocity distribution, and the local pressure distribution as the object to summarize the position angle, the shock velocity, and the bubble distance on the collapse law and the primary and secondary influence relationship and then reveals the interbubble mechanism. The results show that with the increase of the position angle, the collapse velocity of the right side of bubble A gradually decreases compared with the left side, while bubble B has the opposite characteristics. When the angle is 0°, bubble A and bubble B collapse at the same time and the direction of the jet is the same as the shock direction. With the increase of the position angle, the direction of the jet is biased toward bubble B. The collapse time of bubble B gradually increases with the increase in the bubble distance. Taking the collapse time as the evaluation standard, the relationship between the three factors is shock velocity (u) > position angle (θ) > bubble distance (L). In this paper, it perfects the cavitation theory and provides technical support for the efficient application of hydrodynamic cavitation technology.
基于自由域粗粒度力场的双气泡崩塌过程多因素分析
由于气泡破裂所产生的强烈的力学、化学和热效应,空化现象一直是各领域学者研究的热点。它形成一簇气泡,气泡之间会相互影响、相互干扰、相互耦合。为掌握影响气泡破裂的主要因素和气泡间机理,本文采用分子动力学模拟结合粗粒度力场对双气泡模型的破裂过程进行了研究,以气泡的动态形状变化、局部速度分布、局部压力分布为对象,总结了气泡的位置角、激波速度、并分析了气泡距离对崩塌规律和主次影响关系,进而揭示了气泡间的作用机理。结果表明:随着位置角的增大,气泡A右侧的崩塌速度相对于左侧逐渐减小,而气泡B则相反。当角度为0°时,气泡A和气泡B同时坍缩,射流方向与激波方向一致。随着位置角的增大,射流方向偏向气泡B,随着气泡距离的增大,气泡B的坍缩时间逐渐增加。以坍塌时间为评价标准,三者之间的关系为冲击速度(u) >;位置角(θ) >;本文完善了空化理论,为水动力空化技术的有效应用提供了技术支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


