Sp3 ameliorated experimental autoimmune encephalomyelitis by triggering Socs3 in Th17 cells
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
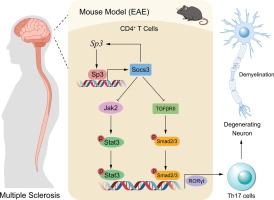
Although it is believed that chronic inflammatory and degenerative diseases of the central nervous system are mediated by autoimmune Th17 cells, the underlying mechanisms remain largely unexplored. Recent studies and our research have revealed that Sp3 was blocked in multiple sclerosis (MS) patients and experimental autoimmune encephalomyelitis (EAE). However, it remained unclear why it is silent and how it regulates Th17 cell differentiation in MS.Objectives
This study aimed to explore the impact of Sp3 on Th17 cell-mediated EAE and the underlying mechanism.Methods
The effect of Sp3 on the clinical symptoms of EAE was evaluated by scoring, histochemistry, and fast blue (FB) techniques, scRNA-seq data analysis, flow cytometry, ELISA, PCR, WB, immunofluorescence and reporter gene techniques were used to explore the molecular mechanism of Sp3 regulating Th17 cell differentiation.Results
Injection of overexpression Sp3 lentivirus could significantly ameliorate the EAE progress and clinical symptoms and prevent the polarization of Th1 and Th17 cells both in vivo and in vitro. We confirmed the occurrence of EAE in Sp3+/+CD4Cre mice and Sp3+/- knockout mice. Furthermore, we identified Sp3 as a target of miR-223, which is found to be upregulated in the blood of MS patients, as well as in EAE and Th17 cells. Moreover, knockdown of miR-223 led to a marked improvement in EAE symptoms and a suppression of Th1 and Th17 cell polarization in vivo and in vitro. Mechanistically, Sp3 significantly suppressed RORγt expression and the phosphorylation of Stat3 and Smad2/3 by directly upregulating Socs3. Interestingly, Socs3 was found to regulate Sp3 expression in response to TGF-β1 via a feedback loop. Moreover, Socs3 modulated phospho-Smad2/3 by binding to and degrading the transforming growth factor-β receptor II (TβRII).Conclusion
Thus, our study suggests a novel mechanism involving miR-223/Sp3/Socs3/TGF-β signaling as a potential therapeutic strategy for targeting Th17 cells in immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


