Piperazine induced enhancement of absorption performance and degradation resistance in biphasic CO2 capture system
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
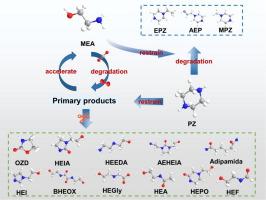
The biphasic solvent has great potential for low-energy CO2 capture. Currently, most studies focus on promoting CO2 absorption performance while ignoring the degradation issue of amines. Here, a novel biphasic solvent was developed, wherein, the ring-structure organic amine with multiple amine groups (piperazine, PZ) was used to simultaneously enhance CO2 absorption performance and degradation resistance of MEA. Results indicated that CO2 absorption capacity increased by 23 % with PZ addition compared to M37B33H30 (M: MEA, B: n-butanol, H: water), while the viscosity and phase separation time were not remarkably affected. Meanwhile, the developed biphasic solvent showed a better degradation resistance than both single MEA and PZ. Specifically, the formation of acid anions was reduced by 66.4 % and 17.4 % compared to single MEA and PZ. It was found that the degradation products of PZ mainly include EPZ, AEP, MPZ. The degradation products of MEA mainly include HEGly, HEPO, HEI, HEA, AEHEIA, Adipamide, BHEOX, and HEEDA, which were significantly inhibited by PZ. The KI can furtherly enhance degradation resistance by removing the dissolved oxygen in the solvent, and the optimal addition amount is 3 %. However, excess KI causes the reduction of Fe3+ to Fe2+, which triggers Fenton reaction to generate hydroxyl radicals (•OH). Finally, a detailed degradation mechanism was proposed by comprehensively analyzing the results of current work and previous studies.
哌嗪诱导提高双相二氧化碳捕获系统的吸收性能和抗降解能力
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


