Dual-functional amino-abundance ultrathin porous boron-doped g-C3N4 co-catalyst for lead halide perovskite-based efficient photocatalytic CO2 reduction
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
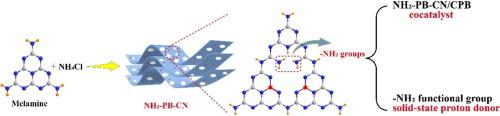
Development of efficient co-catalysts is a crucial strategy to improve photocatalytic CO2 reduction reaction of lead halide perovskites. In this work, dual-functional amino-abundance ultrathin porous boron-doped g-C3N4 (NH2-PB-CN) cocatalyst with solid-state proton donor is developed utilizing melamine as precursor material, NH4Cl as bubble template and H3BO3 as boron-dope source. On the one hand, NH2-PB-CN as co-catalyst forms II-type heterojunction with CsPbBr3, and photogenerated electrons would accumulate on the conduction band of NH2-PB-CN, whose large specific surface area increases the active sites for adsorption and activation of CO2 molecules. Moreover, compared with control g-C3N4 samples, the enhanced reduction potential of NH2-PB-CN also contributes to CO2 conversion. On the other hand, abundant amino groups on NH2-PB-CN as solid-state proton donor provide more protons for CO2 reduction than H2O proton source. To explore the effect of different functional groups on proton supply and CO2 conversion, OH-CN and SO3H-CN as control co-catalysts are synthesized. As a result, product yield of MF/NH2-PB-CN/CsPbBr3 reaches 103.21 μmol/g/h, which is 2.5, 1.1 and 1.7 times higher than those of MF/CN/CsPbBr3 with H2O as proton source, MF/OH-CN/CsPbBr3 and MF/SO3H-CN/CsPbBr3 with solid-state proton donors, respectively, suggesting the effective proton supply from amino groups. MF/NH2-PB-CN/CsPbBr3 also exhibits strong stability and sufficient proton supply, with no obvious yield decrease after four 8-h cyclic measurements.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


