Adsorptive removal of Pb(II) using magnetic MOFs-modified chitosan composite: Preparation, performance and mechanism
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
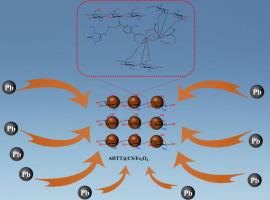
In this work, a novel magnetic composite adsorbent (AHTT@CS/Fe3O4) was synthesized by modifying NH2-MIL-125(Ti) with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (AHTT) and cross-linking it with chitosan (CS) onto a Fe3O4 magnetic core. Structural characterization revealed a porous architecture enriched with active functional groups ( NH2,
NH2,  SH), providing abundant adsorption sites for Pb(II) ions. The composite demonstrated a high theoretical adsorption capacity of 791.36 mg/g at pH 6, with adsorption behavior following the pseudo-second-order kinetic and Hill isotherm models, suggesting a synergistic mechanism driven by multi-site interactions. Thermodynamic analyses indicated a spontaneous and exothermic process, while the material maintained excellent selectivity for Pb(II) even in the presence of competing ions. Reusability tests confirmed its practical robustness, retaining over 80 % removal efficiency after five adsorption–desorption cycles. These results underscore the potential of AHTT@CS/Fe3O4 as a highly efficient, selective, and reusable adsorbent for the remediation of lead-contaminated wastewater.
SH), providing abundant adsorption sites for Pb(II) ions. The composite demonstrated a high theoretical adsorption capacity of 791.36 mg/g at pH 6, with adsorption behavior following the pseudo-second-order kinetic and Hill isotherm models, suggesting a synergistic mechanism driven by multi-site interactions. Thermodynamic analyses indicated a spontaneous and exothermic process, while the material maintained excellent selectivity for Pb(II) even in the presence of competing ions. Reusability tests confirmed its practical robustness, retaining over 80 % removal efficiency after five adsorption–desorption cycles. These results underscore the potential of AHTT@CS/Fe3O4 as a highly efficient, selective, and reusable adsorbent for the remediation of lead-contaminated wastewater.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


