Biochar matrix anchoring pure phase Fe3C to promote advanced oxidation: A reliable pathway for organic wastewater purification
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
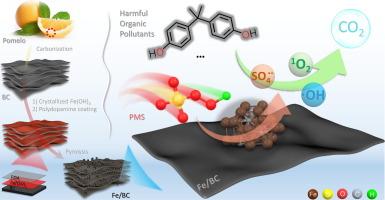
Advanced oxidation process (AOPs) have a significant effect on eliminating harmful organic pollutants including bisphenol A (BPA) in water, but their effectiveness depends on the input of highly active catalysts. Here, a two-dimensional biochar platform was built using inexpensive pomelo mesocarp biomass as raw material, and a highly efficient AOPs catalyst (Fe/BC) with uniform dispersion of iron species was fabricated by surface crystallization, surface encapsulation, and pyrolysis technology. The Fe/BC-PMS (PMS: peroxymonosulfate) system can degrade up to 95.4 % of BPA in 30 min, and the mineralization rate up to 65.8 %, and still maintain about 80 % performance after repeated use for six times. The Fe/BC-PMS system can degrade BPA in complex water environments, including acidic and alkaline conditions, solutions containing coexisting cations and anions, and different water qualities. Continuous and efficient purification of organic pollutants was realized by mixing Fe/BC with quartz sand as the key component of a purification column, demonstrating the potential of Fe/BC in practical applications. Electrochemical signal response combined with radical capture and monitoring confirmed that Fe/BC drives PMS to convert into ·OH, SO4·- and 1O2, which are key reactive oxygen species that promote BPA degradation. Density functional theory (DFT) calculations reveal that the Fe3C site in Fe/BC can spontaneously adsorb PMS and activate O–O bond, which is the key to promoting the conversion of PMS into ROS. These findings offer a crucial experimental and theoretical foundation for the development of advanced AOPs catalysts, presenting cost-effective and efficient solutions for the degradation of harmful organic pollutants.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


