Adult bi-paternal offspring generated through direct modification of imprinted genes in mammals
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Imprinting abnormalities pose a significant challenge in applications involving embryonic stem cells, induced pluripotent stem cells, and animal cloning, with no universal correction method owing to their complexity and stochastic nature. In this study, we targeted these defects at their source—embryos from same-sex parents—aiming to establish a stable, maintainable imprinting pattern de novo in mammalian cells. Using bi-paternal mouse embryos, which exhibit severe imprinting defects and are typically non-viable, we introduced frameshift mutations, gene deletions, and regulatory edits at 20 key imprinted loci, ultimately achieving the development of fully adult animals, albeit with a relatively low survival rate. The findings provide strong evidence that imprinting abnormalities are a primary barrier to unisexual reproduction in mammals. Moreover, this approach can significantly improve developmental outcomes for embryonic stem cells and cloned animals, opening promising avenues for advancements in regenerative medicine.
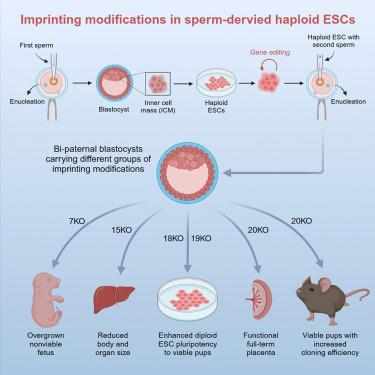
在哺乳动物中通过直接修饰印迹基因而产生的成年双父系后代
印迹异常由于其复杂性和随机性,在胚胎干细胞、诱导多能干细胞和动物克隆的应用中没有通用的纠正方法。在这项研究中,我们将这些缺陷定位于它们的来源——来自同性父母的胚胎——旨在在哺乳动物细胞中建立一种稳定、可维持的印迹模式。利用具有严重印迹缺陷且通常无法存活的双父系小鼠胚胎,我们在20个关键印迹位点引入移码突变、基因缺失和调控编辑,最终实现了完全成年动物的发育,尽管存活率相对较低。这些发现提供了强有力的证据,表明印记异常是哺乳动物单性繁殖的主要障碍。此外,这种方法可以显著改善胚胎干细胞和克隆动物的发育结果,为再生医学的进步开辟了有希望的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


