A cis-regulatory element controls expression of histone deacetylase 9 to fine-tune inflammasome-dependent chronic inflammation in atherosclerosis
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Common genetic variants in a conserved cis-regulatory element (CRE) at histone deacetylase (HDAC)9 are a major risk factor for cardiovascular disease, including stroke and coronary artery disease. Given the consistency of this association and its proinflammatory properties, we examined the mechanisms whereby HDAC9 regulates vascular inflammation. HDAC9 bound and mediated deacetylation of NLRP3 in the NACHT and LRR domains leading to inflammasome activation and lytic cell death. Targeted deletion of the critical CRE in mice increased Hdac9 expression in myeloid cells to exacerbate inflammasome-dependent chronic inflammation. In human carotid endarterectomy samples, increased HDAC9 expression was associated with atheroprogression and clinical plaque instability. Incorporation of TMP195, a class IIa HDAC inhibitor, into lipoprotein-based nanoparticles to target HDAC9 at the site of myeloid-driven vascular inflammation stabilized atherosclerotic plaques, implying a lower risk of plaque rupture and cardiovascular events. Our findings link HDAC9 to atherogenic inflammation and provide a paradigm for anti-inflammatory therapeutics for atherosclerosis.
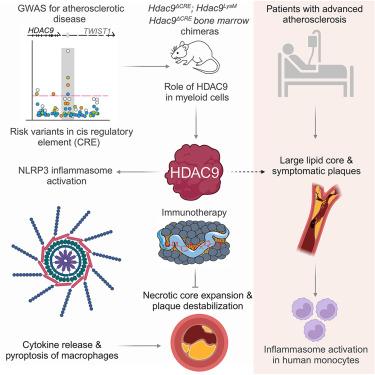
一种顺式调控元件控制组蛋白去乙酰化酶9的表达,以微调动脉粥样硬化中炎性小体依赖性慢性炎症
组蛋白去乙酰化酶(HDAC)9保守顺式调控元件(CRE)的常见遗传变异是心血管疾病(包括中风和冠状动脉疾病)的主要危险因素。鉴于这种关联的一致性及其促炎特性,我们研究了HDAC9调节血管炎症的机制。HDAC9结合并介导NLRP3在NACHT和LRR结构域的去乙酰化,导致炎性体激活和溶解性细胞死亡。在小鼠中靶向删除关键的CRE会增加髓细胞中Hdac9的表达,从而加剧炎症小体依赖性慢性炎症。在人颈动脉内膜切除术样本中,HDAC9表达增加与动脉粥样硬化进展和临床斑块不稳定有关。将IIa类HDAC抑制剂TMP195掺入基于脂蛋白的纳米颗粒中,靶向髓细胞驱动的血管炎症稳定的动脉粥样硬化斑块部位的HDAC9,这意味着斑块破裂和心血管事件的风险较低。我们的研究结果将HDAC9与动脉粥样硬化炎症联系起来,并为动脉粥样硬化的抗炎治疗提供了一个范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


