Redirecting glucose flux during in vitro expansion generates epigenetically and metabolically superior T cells for cancer immunotherapy
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
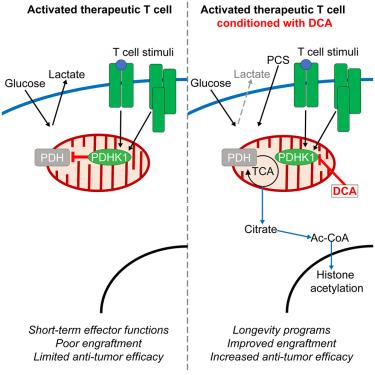
Cellular therapies are living drugs whose efficacy depends on persistence and survival. Expansion of therapeutic T cells employs hypermetabolic culture conditions to promote T cell expansion. We show that typical in vitro expansion conditions generate metabolically and functionally impaired T cells more reliant on aerobic glycolysis than those expanding in vivo. We used dichloroacetate (DCA) to modulate glycolytic metabolism during expansion, resulting in elevated mitochondrial capacity, stemness, and improved antitumor efficacy in murine T cell receptor (TCR)-Tg and human CAR-T cells. DCA-conditioned T cells surprisingly show no elevated intratumoral effector function but rather have improved engraftment. DCA conditioning decreases reliance on glucose, promoting usage of serum-prevalent physiologic carbon sources. Further, DCA conditioning promotes metabolic flux from mitochondria to chromatin, resulting in increased histone acetylation at key longevity genes. Thus, hyperglycemic culture conditions promote expansion at the expense of metabolic flexibility and suggest pharmacologic metabolic rewiring as a beneficial strategy for improvement of cellular immunotherapies.
在体外扩增过程中重定向葡萄糖通量产生表观遗传和代谢优越的T细胞用于癌症免疫治疗
细胞疗法是活体药物,其疗效取决于持久性和存活期。治疗性T细胞的扩增采用高代谢培养条件来促进T细胞扩增。我们发现,典型的体外扩增条件产生代谢和功能受损的T细胞比体内扩增的T细胞更依赖于有氧糖酵解。我们在小鼠T细胞受体(TCR)-Tg和人CAR-T细胞中使用二氯乙酸(DCA)调节扩增过程中的糖酵解代谢,从而提高线粒体容量、干细胞性和抗肿瘤功效。令人惊讶的是,dca条件T细胞在肿瘤内没有表现出升高的效应功能,而是有改善的植入。DCA调节减少了对葡萄糖的依赖,促进了血清中普遍存在的生理性碳源的使用。此外,DCA调节促进了从线粒体到染色质的代谢通量,导致关键长寿基因组蛋白乙酰化增加。因此,高血糖培养条件以牺牲代谢灵活性为代价促进扩张,并提示药理学代谢重新布线是改善细胞免疫治疗的有益策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


