Experimental adaptation to singular pathogen challenge reduces susceptibility to novel pathogens in Drosophila melanogaster
IF 2.7
Q1 ENTOMOLOGY
引用次数: 0
Abstract
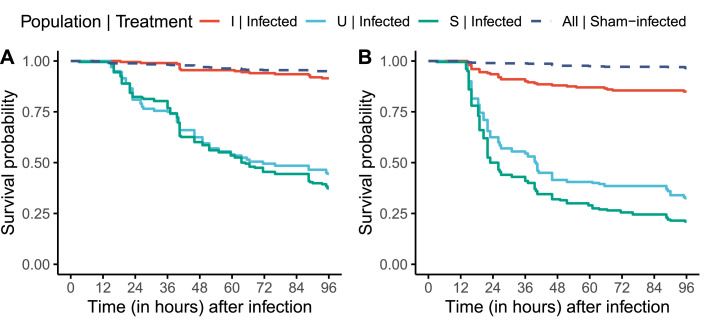
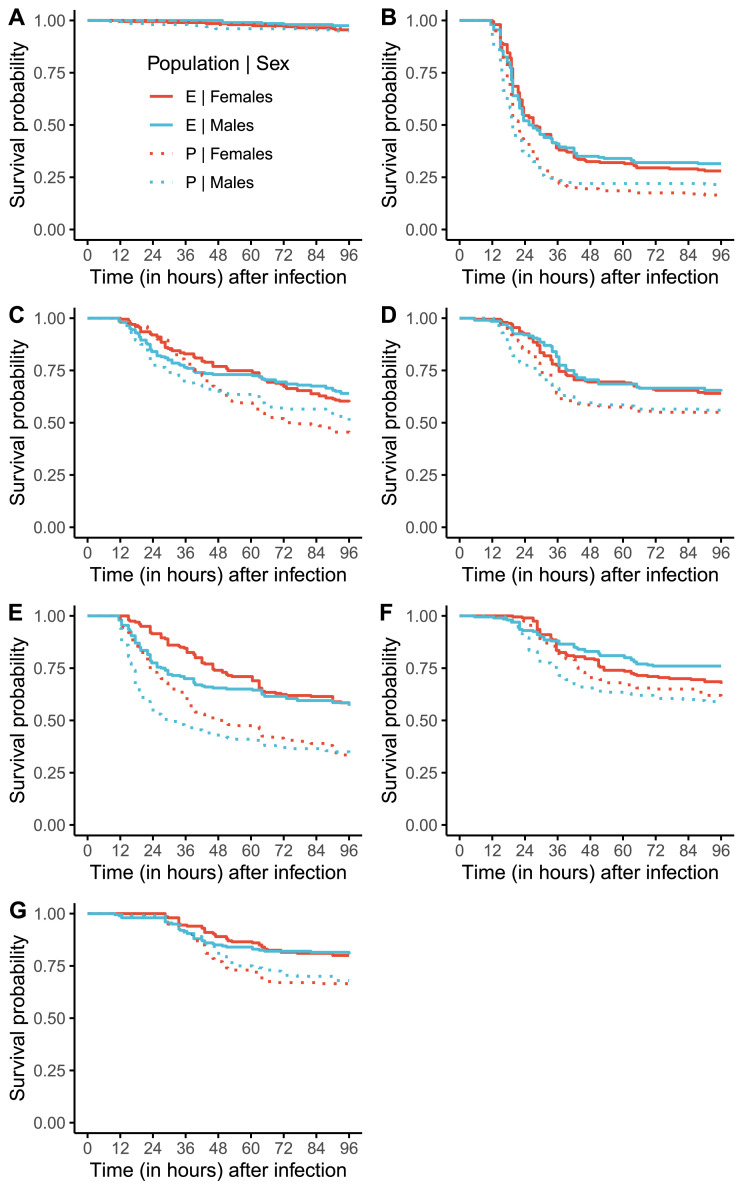
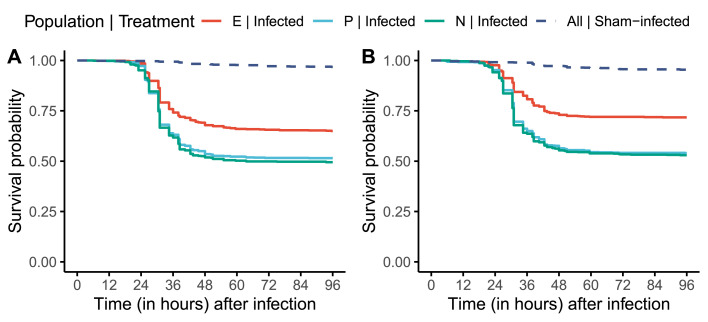
Hosts often encounter and must respond to novel pathogens in the wild, that is pathogens that they have not encountered in recent evolutionary history, and therefore are not adapted to. How hosts respond to these novel pathogens and the outcome of such infections can be shaped by the host's evolutionary history, especially by how well adapted the host is to its native pathogens, that is pathogens they have evolved with. Host adaptation to one pathogen can either increase its susceptibility to a novel pathogen, due to specialization of immune defenses and trade-offs between different arms of the immune system, or can decrease susceptibility to novel pathogens by virtue of cross-resistance. Using laboratory Drosophila melanogaster populations, we explore if hosts experimentally adapted to surviving infection challenges by a single bacterial pathogen are also better at surviving infection challenges by novel bacterial pathogens. We found that such hosts can survive infection challenges by multiple novel pathogens, with the expanse of cross-resistance determined by the identity of the native pathogen and sex of the host. Therefore, we have demonstrated that cross-resistance can evolve in host populations by virtue of adaptation to a single pathogen. This observation has important ecological consequences, especially in the modern era where spillover of novel pathogens is a common occurrence due to various factors, including climate change.
对单一病原体挑战的实验适应降低了果蝇对新病原体的敏感性。
宿主经常在野外遇到并且必须对新的病原体作出反应,这些病原体是它们在最近的进化历史中没有遇到的,因此不适应的病原体。宿主对这些新型病原体的反应以及这种感染的结果可以由宿主的进化史来决定,特别是宿主对其原生病原体的适应程度,也就是与它们一起进化的病原体。宿主对一种病原体的适应可以增加其对新病原体的易感性,这是由于免疫防御的专门化和免疫系统不同分支之间的权衡,或者可以通过交叉抗性降低对新病原体的易感性。利用实验室黑腹果蝇种群,我们探索了宿主是否在实验上适应了单一细菌病原体的感染挑战,也更好地适应了新型细菌病原体的感染挑战。我们发现,这些宿主可以在多种新病原体的感染挑战下存活下来,其交叉抗性的范围取决于本地病原体的身份和宿主的性别。因此,我们已经证明,交叉抗性可以在宿主种群中通过适应单一病原体而进化。这一观察结果具有重要的生态后果,特别是在现代,由于包括气候变化在内的各种因素,新型病原体的外溢是一种常见现象。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Insect Science
Agricultural and Biological Sciences-Animal Science and Zoology
CiteScore
3.20
自引率
0.00%
发文量
22
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


