An efficient direct electrolysis method for the synthesis of 1,1,1,3,3,3-hexafluoroisopropyxy substituted imidazo[1,2-a]pyridines†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Electrochemical oxidative cross-dehydrogenative-coupling (CDC) is an ideal strategy to conduct the C3-alkoxylation of imidazo[1,2-a]pyridine, but it remains a challenge owing to limitation imposed by the use of alkyl alcohols and carboxylic acids. Herein, we report a mild and efficient 2-electrode constant-potential electrolysis of imidazo[1,2-a]pyridine with hexafluoroisopropanol (HFIP) to produce various imidazo[1,2-a]pyridine HFIP ethers. Mechanistic studies indicated that the electrooxidation reaction might involve radical coupling and ionic reaction.
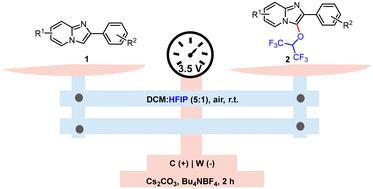
直接电解法合成1,1,1,3,3,3-六氟异丙基取代咪唑[1,2-a]吡啶。
电化学氧化交叉脱氢偶联(CDC)是进行咪唑[1,2-a]吡啶的c3 -烷氧基化的理想策略,但由于使用烷基醇和羧酸的限制,它仍然是一个挑战。本文报道了咪唑[1,2-a]吡啶与六氟异丙醇(HFIP)进行温和、高效的双电极恒电位电解,以生产各种咪唑[1,2-a]吡啶HFIP醚。机理研究表明,电氧化反应可能包括自由基偶联反应和离子反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


