Clinical perspective on pluripotent stem cells derived cell therapies for the treatment of neurodegenerative diseases
IF 15.2
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Self-renewal capacity and potential to differentiate into almost any cell type of the human body makes pluripotent stem cells a valuable starting material for manufacturing of clinical grade cell therapies. Neurodegenerative diseases are characterized by gradual loss of structure or function of neurons, often leading to neuronal death. This results in gradual decline of cognitive, motor, and physiological functions due to the degeneration of the central nervous systems. Over the past two decades, comprehensive preclinical efficacy (proof-of-concept) and safety studies have led to the initiation of First-in-Human phase I-II clinical trials for a range of neurodegenerative diseases. In this review, we explore the fundamentals and challenges of neural-cell therapies derived from pluripotent stem cells for treating neurodegenerative diseases. Additionally, we highlight key preclinical investigations that paved the way for regulatory approvals of these trials. Furthermore, we provide an overview on progress and status of clinical trials done so far in treating neurodegenerative diseases such as spinal cord injury (SCI), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), as well as advances in retina diseases such as Stargardt disease (a.k.a fundus flavimaculatus), retinitis pigmentosa (RP) and age-related macular degeneration (AMD). These trials will pave the way for the development of new cell-based therapies targeting additional neurological conditions, including Alzheimer’s disease and epilepsy.

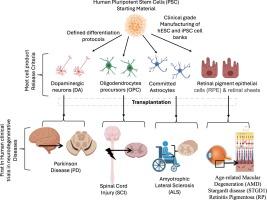
多能干细胞衍生细胞疗法治疗神经退行性疾病的临床前景
多能干细胞具有自我更新能力和分化成人体几乎所有细胞类型的潜力,因此是制造临床级细胞疗法的宝贵起始材料。神经退行性疾病的特点是神经元的结构或功能逐渐丧失,往往导致神经元死亡。中枢神经系统的退化会导致认知、运动和生理功能的逐渐衰退。在过去的二十年里,通过全面的临床前疗效(概念验证)和安全性研究,针对一系列神经退行性疾病的 I-II 期临床试验相继启动。在本综述中,我们将探讨多能干细胞神经细胞疗法治疗神经退行性疾病的基本原理和挑战。此外,我们还重点介绍了为这些试验获得监管部门批准铺平道路的关键临床前研究。此外,我们还概述了迄今为止在治疗脊髓损伤(SCI)、帕金森病(PD)和肌萎缩侧索硬化症(ALS)等神经退行性疾病方面所做临床试验的进展和现状,以及在治疗斯塔加特病(又称眼底黄斑病变)、视网膜色素变性(RP)和老年性黄斑变性(AMD)等视网膜疾病方面取得的进展。这些试验将为开发针对更多神经系统疾病(包括阿尔茨海默病和癫痫)的新型细胞疗法铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


