A Ni2P/NiMoOx nanocone electrocatalyst for efficient hydrogen evolution: tip-enhanced local electric field effect
IF 5.8
3区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The sluggish kinetics of the hydrogen evolution reaction (HER) result in a high overpotential in alkaline solutions. A high-curvature metal oxide heterostructure can effectively boost the electrocatalytic HER by leveraging the tip-enhanced local electric field effect. Herein, Ni2P/NiMoOx nanocones were synthesised on a nickel foam (NF) substrate by etching a metal–organic framework template. The Ni2P/NiMoOx nanocones on the NF substrate served as an advanced electrocatalyst for the HER. Analysis using the finite element method indicated that the high-curvature tips of the Ni2P/NiMoOx nanocones enhanced the local electric field, resulting in a higher concentration of hydrated K+ ions (K(H2O)6+), which facilitated water dissociation and accelerated the reaction kinetics. The tip-enhanced local electric field effect accelerates the mass transfer rate, and the heterostructure promotes charge transfer to activate the active center, thereby synergically enhancing the electrocatalytic reaction. The Ni2P/NiMoOx nanocone electrocatalyst exhibited low overpotentials of 49, 137 and 274 mV at 10, 100 and 500 mA cm−2, respectively, under alkaline conditions for the HER. In addition, the electrocatalyst demonstrated excellent stability over 200 h at 300 mA cm−2. This study provides a promising approach for developing efficient electrocatalysts that facilitate the HER in alkaline solutions.
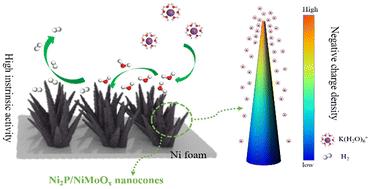
氢进化反应(HER)的缓慢动力学导致其在碱性溶液中具有很高的过电位。高曲率金属氧化物异质结构可利用尖端增强的局部电场效应,有效促进电催化氢催化反应。在此,通过蚀刻金属有机框架模板,在泡沫镍(NF)基底上合成了 Ni2P/NiMoOx 纳米锥。NF基底上的Ni2P/NiMoOx纳米锥体是一种先进的热释电催化剂。利用有限元法进行的分析表明,Ni2P/NiMoOx 纳米锥的高曲率尖端增强了局部电场,导致水合 K+ 离子(K(H2O)6+)浓度升高,从而促进了水的解离并加速了反应动力学。尖端增强的局部电场效应加快了传质速率,异质结构促进了电荷转移以激活活性中心,从而协同增强了电催化反应。在碱性条件下,Ni2P/NiMoOx 纳米锥电催化剂在 10、100 和 500 mA cm-2 的过电位分别为 49、137 和 274 mV。此外,该电催化剂在 300 mA cm-2 的条件下可稳定工作 200 小时。这项研究为开发促进碱性溶液中 HER 的高效电催化剂提供了一种可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nanoscale
CHEMISTRY, MULTIDISCIPLINARY-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
12.10
自引率
3.00%
发文量
1628
审稿时长
1.6 months
期刊介绍:
Nanoscale is a high-impact international journal, publishing high-quality research across nanoscience and nanotechnology. Nanoscale publishes a full mix of research articles on experimental and theoretical work, including reviews, communications, and full papers.Highly interdisciplinary, this journal appeals to scientists, researchers and professionals interested in nanoscience and nanotechnology, quantum materials and quantum technology, including the areas of physics, chemistry, biology, medicine, materials, energy/environment, information technology, detection science, healthcare and drug discovery, and electronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


