Chloridion induced acid sites in covalent organic frameworks for 5‑hydroxymethylfurfural synthesis from fructose
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
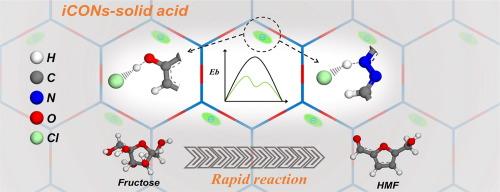
The versatile architecture of covalent organic frameworks (COFs) provides a powerful platform for tailoring their functions. Herein, we demonstrate the molecular engineering of 2D ionic COF nanosheets (iCONs) to reach a family of organic polymeric catalysts with tunable acidity. These solid acidic iCONs are synthesized through Schiff base condensation of the ionic monomer triaminoguanidinium chloride and the aromatic aldehydes with different surface groups. Compared with that in the monomer, the Cl– in iCON matrix tends to be near the framework H atom, generating a new Brønsted acid site with much short Cl–∼H+ distance that resembles HCl. As a result, these iCONs are highly active in the typical acid reactions of aldol condensation and dehydration of fructose into 5-hydroxymethylfurfural (HMF). The shorter Cl–∼H+ distance, the better acid catalytic activity. The catalyst DHPA-TGCl reaches a high HMF yield of above 97 % within a short reaction time of 15 min, providing the turnover frequency (TOF) as high as 155.2 h−1. Facile recycling and stable reusability are also observed. The free energy profiles of these iCONs catalyzing fructose conversion to HMF confirm the function of Cl–∼H+ units in lowering the energy barrier of the rate-determining step for the water release in the HMF synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


