Synthesis and Resolution of 4′-Substituted Nucleosides with Potential Antiviral and Antisense Strategies
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
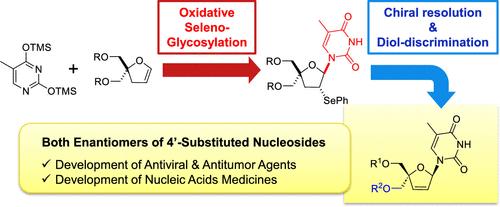
Nucleoside derivatives having a 4′-substituent show promise as potential antiviral agents as well as nucleoside units for constructing nucleic acid medicines. To develop new nucleosides, it is crucial to achieve feasible access to the intended derivatives, encompassing both enantiomers. Toward this end, we started synthesizing an achiral 4-hydroxymethyldihydrofuran as a sugar precursor, which we subjected to the oxidative glycosylation reaction using hypervalent iodine. The resulting racemate of a 4′-hydroxymethylated thymidine derivative underwent kinetic resolution using lipase, yielding both d- and l-isomers with high optical purity. The d-4′-hydroxymethylstavudine derivative was then converted into the corresponding phosphoramidite derivative, from which an oligonucleotide was synthesized.
具有潜在抗病毒和反义策略的4′-取代核苷的合成和拆分
具有 4′-取代基的核苷衍生物有望成为潜在的抗病毒药物以及构建核酸药物的核苷单元。要开发新的核苷类药物,就必须能够获得包括两种对映体在内的预期衍生物。为此,我们开始合成无手性的 4-羟甲基二氢呋喃作为糖前体,并使用高价碘对其进行氧化糖基化反应。由此产生的 4′-羟甲基化胸苷衍生物的外消旋体利用脂肪酶进行了动力学解析,得到了高光学纯度的 d-和 l-异构体。然后将 d-4′-hydroxymethylstavudine 衍生物转化为相应的亚磷酰胺衍生物,并以此合成寡核苷酸。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


