Photoresponsive Bio-Heterojunctions Eliciting Immunogenicity to Prevent Infection Recurrence and Accelerating Chronic Wound Regeneration
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
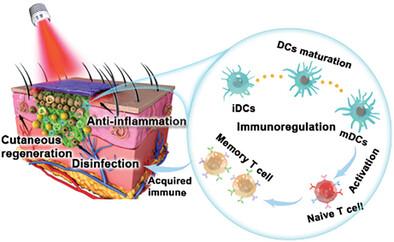
Dynamic therapy utilizes reactive oxygen species (ROS) to antibacterial and enhance the innate immune system to treat bacterial infections. If ROS levels are too low, the elimination of pathogens and the enhancement of innate immunity cannot be achieved. However, excess accumulation of ROS may impact intracellular glutathione (GSH) levels, hindering T cell maturation and the establishment of immune memory. Herein, a multifunctional nanofiber membrane is designed, consisting of a polymer scaffold, MXene/CeO2 bio-heterojunctions (MX@Ce bio-HJs), and lactate oxidase (Lox) to balance the production of ROS, for the treatment of recurrent bacterial infections. In this system, MX@Ce bio-HJs upon near-infrared ray (NIR) generate photodynamic therapy, while Lox responds to the wound microenvironment exert chemodynamic therapy, synergistically produce ROS to rapidly eradicate bacteria, amplify the ability of dendritic cells to recognize and present antigens of bacterial fragments, and enhance innate immunity. Without NIR, MX@Ce bio-HJs showcase catalase-like and superoxide dismutase-like activities, scavenging subsequent ROS accumulation, promoting T cell maturation to form acquired immune memory, and combating recurrent bacterial infection. Such work highlights the potential to combat in situ bacterial infections and recurrent bacterial infections and inspires the development of future antibacterial therapies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


