Unraveling the contribution of acidic metal oxides modulating the CeO2/TiO2 catalyst acid sites for NH3-SCR activity and SO2 tolerance
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
As a potential substitute for V2O5-WO3/TiO2 (V-W/Ti) catalyst, CeO2/TiO2 (Ce/Ti) catalyst still faces severe challenges regarding low-temperature denitrification performance and SO2 tolerance. To this end, we report an effective strategy to modulate Ce/Ti catalyst acid sites by different acidic metal oxides. The modification of Nb, Mo and W species can well weaken the L-acid sites and enhance the B-acid sites of Ce/Ti catalyst, which helps more NH3 adsorb to form the ionic NH4+ for activation at low temperatures. Moreover, the improvement of redox performance well weakens the adsorption of low reactive nitrates and facilitates the NO oxidation of NO2. The optimum sample (Ce-Nb/Ti) achieved 95 % NOx conversion within the active temperature window (225 − 425 °C) and admirable SO2-tolerant performance at 300 °C. The abundant surface acidity and strong interactions between metal ions not only suppress the adsorption of SO2 on the Ce-Nb/Ti but also decrease the thermal stability of ammonia bisulfate and alleviate sulfate deposition. Moreover, the in situ DRIFTS experiments demonstrated that introducing these acidic metal oxides on Ce/Ti catalyst did not change the reaction pathway, following the Langmuir-Hinshelwood and Eley-Rideal mechanisms.
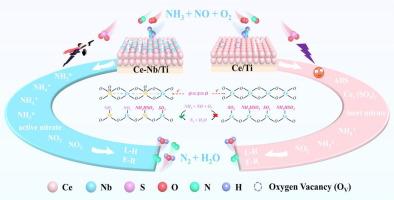
揭示酸性金属氧化物调节 CeO2/TiO2 催化剂酸性位点对 NH3-SCR 活性和二氧化硫耐受性的贡献
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


