A Coordination Nanosystem Enables Endogenous Ferric Ion-Initiated Multi-Catalysis for Synergistic Tumor-Specific Ferroptosis and Gene Therapy
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
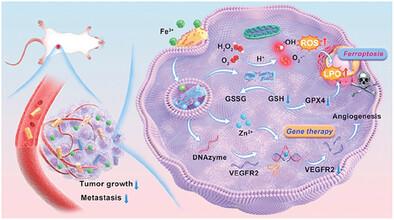
Emerging evidence demonstrates that inducing ferroptosis, a nonapoptotic programmed cell death mode, holds significant potential for tumor treatment. However, current ferroptosis strategies utilizing exogenous Fenton-type heavy metal species or introducing glutathione (GSH)/glutathione peroxidase 4 (GPX4) suppressants are hampered by latent adverse effects toward organisms, while utilizing endogenous iron may cause undesirable tumor angiogenesis through specific signaling pathways. Here, a ferric ion (Fe3+)-responsive and DNAzyme-delivered coordination nanosystem (ZDD) is developed to achieve a novel scheme of synergistic tumor-specific ferroptosis and gene therapy, which modulates and harnesses the endogenous iron in tumors for inducing ferroptosis while intercepting tumor angiogenesis to enhance therapeutic efficacy. Profiting from the characteristic coordination structure and components, ZDD can not only specifically capture tumor endogenous Fe3+ into the tumor cells to promote the catalytic generation of hydroxyl radicals (·OH) and superoxide anions (O2·−) under acidic environment and the catalytic GSH oxidation, arousing potent ferroptotic cell death, but also effectively deliver specific DNAzyme and release abundant zinc ion (Zn2+) as a powerful cofactor to activate the biocatalytic cleavage of vascular endothelial growth factor receptor 2 (VEGFR2) gene for angiogenesis suppression. Ultimately, the ZDD-enabled synergistic therapy prominently inhibited tumor growth and prevented metastasis, representing a promising nanotherapeutic formula for safe and efficient tumor therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


