Revealing the reversible mechanism of lithium hydride and its role in accelerating graphite anode failure
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
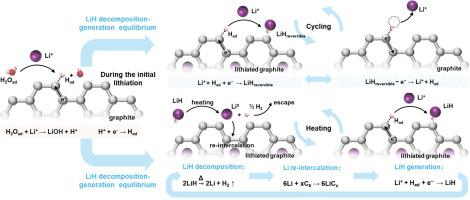
The generation of LiH and its decomposition products such as H2 pose safety concerns for lithium ion batteries. However, the reversible mechanism underlying LiH formation and its impact on battery cycling stability remain unclear. Here, we quantify the formation and evolution of LiH on practical graphite anodes during cycling. It is disclosed for the first time that reversible LiH formation/removal on the graphite anode during lithiation/delithiation even wherein there is no Li metal plating. By comparing LiH formed with chemically synthesizing LiCx, theoretical simulations and a series of spectroscopic results, we propose a new formation mechanism of LiH which is induced by highly lithiated graphite such as LiC6. For the reversibly formed LiH, the Li and H element comes from Li+ in graphite and H2O adsorbed on graphite surface, respectively. In addition, we conducted the heating-mass spectrometry titration (H-MST) experiment to quantify its thermal stability at 60 °C, unveiling a dynamic equilibrium between LiH formation and decomposition, e.g. metallic Li resulting from LiH decomposition can re-intercalate into graphite, leading to the regeneration of LiH and further production of H2 supposed with rich-sourced hydrogen species from the electrolytes. The continuous accumulation of H2 poses a potential safety hazard for a long-term cycling of LIBs. We believe that this new discovery on LiH in this work provides a unique insight for the understanding and managing of thermal safety of LIBs caused by existences of hydrogen.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


