Dynamics of Hydrogen-bonded Polymer Complexes of Poly(ether oxide) and Poly(acrylic acid): Time-Humidity-Temperature Equivalence
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
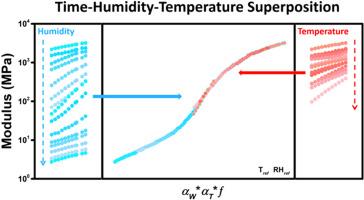
The dynamics of polymer complexes over long-time scales are critical for their applications across various fields. Humidity is a very important environmental parameter and its coupling with temperature significantly affects the performance of polymer complex materials. However, the understanding of humidity effect on hydrogen-bonded polymer complex is poor and the quantitative studies are lacking. Here, we investigate the static and dynamic mechanical properties of hydrogen-bonded polymer complexes of poly(ethylene oxide) (PEO) and poly(acrylic acid) (PAA) and its reinforced derivatives under different humidity and temperature. When PEO/PAA complexes are reinforced through covalent cross-linking and coordination, both the humidity-induced glass transition and the glass transition temperature (Tg) increase. The equilibrium water uptake as a function of relative humidity (RH) shows two distinct trends, and the trend transition corresponds to the humidity-induced glass transition. Time-humidity equivalence and time-temperature equivalence are applied separately to construct the master curves. The temperature-dependent shift factor (aT) is fitted to Arrhenius and Williams-Landel-Ferry (WLF) equations before and after Tg, respectively. Instead of the value of RH, the equilibrium water ratio in the polymer complex is used to describe humidity-dependent shift factor (aW), which fits two log-linear equations before and after humidity-induced glass transition. Furthermore, we combine humidity and temperature to do superposition to build the master curve of much longer timescale, and propose a coefficient (fc) to describe how the temperature and humidity working together and coupling effect.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


