Anti-tumor Effects of Artemisinin-Based Oligomers: From Monomer to Trimer as a Novel Drug-Enhancing Strategy
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
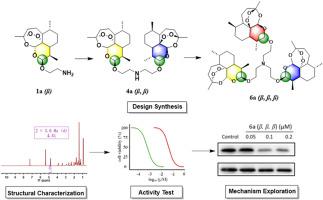
Artemisinin and its derivatives (ARTs) are being studied for their potential anti-tumor activity. Dimerization of artemisinin has been proposed as a promising means of enhancing drug efficacy. However, the sequential progression from monomers to dimers and trimers, retaining a consistent β-configuration, has not been previously investigated in terms of its effect on compound activity. To investigate the effect of various oligomeric forms on drug potency, we synthesized β-configuration-based ARTs, namely a monomer, dimer, and trimer, and rigorously characterized their structure. We evaluated the antitumor efficacy of these compounds against MCF-7 breast cancer cells. The artemisinin trimer 6a, (β, β, β) exerted a stronger cytotoxic effects against MCF-7 breast cancer cells, with an IC50 value of 0.09 ± 0.03 μM, than did the monomer (β) or dimer (β, β), which had IC50 values of >50 and 3.14 ± 0.54 μM, respectively. This specific configuration induced alterations in nuclear morphology, inhibited colony formation, and facilitated cancer cell death. Mechanistic studies revealed that 6a (β, β, β) promoted apoptosis by modulating the Bax-caspase 3 signaling pathway and induced ferroptosis by regulating key signaling molecules, including GPX4. This study introduces an innovative methodology—a stepwise synthesis strategy progressing from monomers to dimers and trimers—to explore the relationship between oligomeric structure and drug activity. These findings provide novel insight into the architecture–activity relationship of ART derivatives, offering a foundation for advancing drug design and improving clinical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


