Computer-aided discovery of novel AMPK activators through virtual screening and SAR-driven synthesis
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
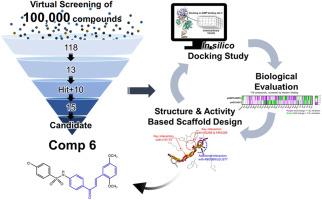
AMPK is a promising target for various chronic illnesses such as diabetes and Alzheimer's disease (AD). We sought to develop a novel small molecule that directly activates AMPK, with the potential to fundamentally modulate the pathogenic mechanisms of the metabolic disorders. To identify a potent novel pharmacophore in an unbiased way, we performed structure-based virtual screening on a commercially available chemical library, and evaluated the actual AMPK activity of 118 compounds selected from 100,000 compounds based on docking scores. Additional iterative molecular docking studies and experimental evaluation of AMPK activity led us to select a hit compound, B1, with a chromone backbone. Using the hit compound and other compounds structurally similar to the hit compound, we identified the chalcone structure as a new scaffold with more efficient interactions with key residues required for AMPK activation. From the newly designed and synthesized chalcone derivatives, we discovered compound 6 as a candidate compound. Compound 6 showed the most efficient interactions with the key residues of AMPK at in silico study and demonstrated significant activation of AMPK in both in vitro and in cellular assays.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


