Lignosulfonate-based adsorbents for selective Mg2+ removal from Mg2+/Li+ mixture in water with high efficiency and reusability
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
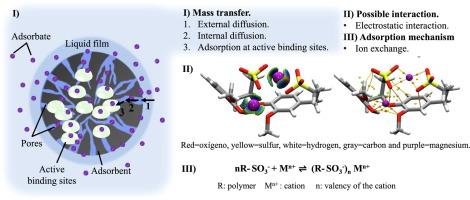
This study focuses on the removal of Mg2+ from aqueous solutions during Li+ recovery. The objective was to capture Mg2+ ions by adsorption using based adsorbents of sulfonated lignin (LS) and poly-3-sulfopropyl potassium acrylate (PSPAK). The bio-based adsorbents were obtained in high mass yields by radical polymerization. Their structural composition and porous morphology were corroborated by infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). Lignin reinforces the mechanical properties of the material by increasing its resistance to deformation under a constant load, making it a suitable choice for adsorption processes. During the experiments, approximately 98.0 % of Mg2+ ions were removed using aqueous solutions with Mg2+/Li+ mass ratios (mg/L) of 0.5/1, significantly reducing the magnesium concentration in the aqueous medium. More than 90.0 % of Li+ was recovered from solution in the presence of Mg2+ at high ratios 8/1 and 10/1. The adsorbents retain their affinity for Mg2+ ions even in the presence of interfering ions such as Li+, Na+, K+, and Ca2+. Furthermore, they can be reused for more than five consecutive adsorption–desorption cycles, without a significant decrease in their adsorption efficiency and structural stability. The computational results indicate that the presence of Mg2+ ions has an impact on the stability and conformational behavior of the system. The polymer-Mg2+/Li+ system demonstrates a stronger affinity for Mg2+ compared to Li+. The increased adsorption energy and greater stability of the polymer-[Mg2+] combination provide evidence for the improved selectivity and effectiveness of polymer-based adsorbents in removing Mg2+ in the presence of Li+.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


