Selective separation of arsenic and antimony enabled by desolvation effect during distillation process: chlorine/oxygen affinity mechanism
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
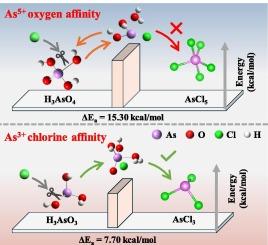
The depth-separation of arsenic from antimony is crucial for achieving high-purity antimony metal. Chlorinated distillation has shown to effectively lower arsenic levels to just a few ppm, making it a promising method for arsenic impurity removal. However, many aspects of the deep separation mechanism of arsenic remain unexplored, posing technical barriers to effective arsenic removal in high-purity antimony production. This work introduces a “chlorine/oxygen affinity selective distillation” model to elucidate the mechanism underlying arsenic and antimony separation in chloride systems. Through thermodynamic analysis and quantum chemistry calculation, we demonstrate that arsenic species in different oxidation states exhibit markedly distinct affinities for chlorine and oxygen. The critical factor is the change in the solvation structure of As3+ as it interacts with Cl−, while the strong binding affinity between As5+ and oxygen inhibits chloride-mediated desolvation. Thus, HCl concentration plays a pivotal role in enhancing the separation efficiency of As3+ from Sb3+. Experimental results reveal that increasing HCl concentration from 5 mol/L to 7 mol/L boosts arsenic removal efficiency by 5.7 %. This mechanism may offer broader applications for the efficient separation of similar elements in chloride systems.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


